Technical Session 4
Emerging Remediation Technologies
Wednesday, November 15, 2023 | 1:00 - 5:00 p.m. Pacific Time
► WATCH THE RECORDING:

Complex sites face a number of remediation challenges related to multiple contaminant types (e.g., inorganics, metals, radionuclides, organics), the depth and heterogeneous nature of the subsurface, variations in flow/boundary conditions over time, and other site characteristics. This session will focus on emerging technologies for in situ remediation to address these types of complex site challenges in the vadose zone (e.g., to treat sources or control flux to groundwater) and in groundwater aquifers. Topics in this session will include technology development (lab-scale, pilot-scale, or full-scale), implementation approaches, including innovative access and delivery methods, and multi-step or combined remediation technologies.
Session Organizers: Jim Szecsody, Pacific Northwest National Laboratory; Holly Vermeulen, Savannah River National Laboratory; Tim Boe, U.S. Environmental Protection Agency; Christian D. Johnson, Pacific Northwest National Laboratory; and Daniel Kaplan, University of Georgia
| 1:00 - 1:05 p.m. |
Opening Remarks __________________________________________________ |
|
1:05 - 1:25 p.m. In Situ Biomineralisation for Sellafield Groundwater Radionuclide Remediation Callum Robinson, The University of Manchester ► PRESENTATION PDF |
Globally, 70+ years of nuclear fuel cycle activities have led to unanticipated discharges of radionuclides to the sub-surface at key sites such as Hanford, USA and Sellafield UK. Where uranium and strontium-90 contamination plumes are present. Developing (co-)remediation strategies for 90Sr and U is therefore important in maintaining the stewardship of these sites in the medium to long term. Calcium phosphate minerals have been suggested as promising radionuclide (including 90Sr and U) sinks. In this study, biotic (calcium citrate/sodium phosphate, glycerol phosphate) and abiotic (polyphosphate) phosphate in-situ amendments were tested using sediment microcosm and flowing column experiments. The aim was to extend the envelope of application of these techniques to Sellafield, UK using relevant sediments and groundwaters. For U(VI) and stable Sr-challenged microcosms, aqueous geochemical results suggest the addition of phosphate-generating amendments enhanced Sr and U removal from solution when compared to the sediment-only sorption controls. After treatment with phosphate amendments microcosm sediment endpoints were taken for solid phase characterisation techniques including SEM and XAS. Here, EXAFS analysis showed evidence for non-crystalline U(VI) phosphate and confirmed some Sr incorporation into Ca-phosphate precipitates. Further investigation was conducted using flowing column studies, designed to represent typical Sellafield groundwater flow regimes. The addition of phosphate generating amendments decreased the rate of Sr breakthrough when compared to a sediment only column. Post treatment, columns were sectioned (bulk EXAFS) and resin embedded (µXRF; INE-beamline, KIT, Germany) to enable solid phase characterisation. Here, bulk EXAFS analysis showed some evidence for Sr incorporated Ca-phosphate phases in treated columns and initial analysis of µXRF data showed hot spots of Sr at the bases of treated columns. This work shows for the first time a comparative assessment of these mineralisation techniques and their end-points and widens the envelope for their application at nuclear facilities. Coauthors: Sam Shaw (The University of Manchester), Jonathan R. Lloyd (The University of Manchester), James Graham (National Nuclear Laboratory), Jörg Rothe (Institute for Nuclear Waste Disposal), Kathy Dardenne (Institute for Nuclear Waste Disposal), Karlsruhe Institute of Technology), Katherine Morris (The University of Manchester) |
|
1:25 - 1:45 p.m. The SURRI Project: Developing New Technologies for Risk Management and Critical Element Recovery at Legacy Uranium Production Sites. Andrew Cundy, University of Southampton (UK) ► PRESENTATION PDF |
Former uranium mining and production facilities present complex remediation and rehabilitation challenges. For uranium mining and milling tailings in particular significant risk may be generated by the presence of (a) elevated radioactivity due to uranium and daughter products, (b) potentially toxic metals and other toxic compounds, and/or (c) sulphide minerals, which may promote acid mine drainage. This, coupled with the often-large spatial coverage of tailings and other wastes, and their susceptibility to leaching, erosion or collapse, means that sizeable areas of land are made unfit for other uses and require risk management or rehabilitation. The SURRI project is a recently initiated Horizon Europe programme aimed at developing new integrated (and more sustainable) risk management methods for legacy uranium mining and production sites (and other sites with uraniferous or thoriated wastes) in Eastern Europe. Led by the Technical University of Liberec in the Czech Republic, the project draws on expertise from partners in Italy, Spain and the UK to develop new electrochemical and biological remediation processes to manage risk from uranium and uranium-daughter products (and co-contaminants) in legacy solid wastes and surface- and ground-waters, while also developing new methods for element recovery from waste solids and leachates / drainage waters. The recovery process focuses particularly on application of low-cost and more sustainable chemical, phyto-based and microbial methods that can be used to recover critical elements (e.g. noble metals, Se, Cu and Ni) in nano-, biomolecule and other forms from wastes, for use in novel green chemical, photoelectronic and other applications. This presentation examines the initial target sites for the SURRI project, their risk management and rehabilitation challenges, and the integrated electrochemical and biological methods under development to (a) promote their rehabilitation, and (b) recover valuable waste products, supporting local and regional supply chains (and the local economy) and potential site re-use. Coauthors: Miroslav Cernik, Alena Sevcu (Technical University of Liberec, Czech Republic), Martin Palusak (Technical University of Liberec, Czech Republic), Veronika Hlavackova (Technical University of Liberec, Czech Republic), Nhung H.A. Nguyen (Technical University of Liberec, Czech Republic), Vira Velianik, Trung Duc Le (Technical University of Liberec, Czech Republic), Cristina Povedano Priego, Mohamed Merroun (University of Granada, Spain), Marco Petrangeli Papini (La Sapienza University, Rome, Italy), Frances Burrell (University of Southampton, UK.), Richard Marsh (University of Southampton, UK.) |
|
1:45 - 2:05 p.m. Synthesis and Characterization of Functionalized Organoclays for Use in a Radioactive Waste Repository Carolyn Pearce, Pacific Northwest National Laboratory ► PRESENTATION PDF |
Performance assessment of nuclear waste disposal options requires implementation of effective buffer materials. Buffer materials must meet longevity requirements and scavenge challenging radioisotopes, e.g., iodine-129 (I-129) and technetium-99 (Tc-99), both long-lived, highly mobile, anionic species in aerobic environments. In many proposed nuclear waste repositories, heat-generating radioactive waste will be surrounded by clay buffer material, which swells to fill the gap between the waste package and the host geology, restricting radionuclide transport to diffusive processes for several hundred years. These favorable properties of clays have led to the planned use of bentonite, which consists predominantly of the dioctahedral smectite, montmorillonite. Bentonite is effective at sequestering cationic fission products, owing to its low zero point of charge, but requires functionalization to change the surface charge for the capture of anionic species, such as pertechnetate (TcO4-), iodide (I-) and iodate (IO4-). Here, we functionalized organoclays with alkylammonium cations of different sizes and different metal cations, then characterized them using x-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), carbon analysis, zeta potential, and surface area measurements. Molecular dynamics (MD) and density functional theory (DFT) calculations calculations were used to inform the orientation of the alkylammonium cations in the clay interlayers. The physicochemical characteristics of the clays were then correlated with the extent of TcO4- removal in batch sorption experiments, with results showing that the order of alkylammonium/metal ion addition has important consequences for Tc-99 removal efficiency. The hydrophobicity of the chains, the hydrophilicity of the ammonium groups, their ability to bind with anions and their affinity with siloxane surfaces necessarily lead to different behaviors in terms of water and anion uptake for the linear and branched alkylammonium. Insight into the competition between the different mechanisms can be gained using MD and DFT. Coauthors: Nathalie A. Wall (University of Florida), Emily Maulden (University of Florida), Elizabeth Gager (University of Florida), Seaton Ullberg (University of Florida), An Ta, Juan C. Nino (University of Florida), Simon Phillpot (University of Florida), Maxime Pouvreau (Pacific Northwest National Laboratory), James E. Szecsody (Pacific Northwest National Laboratory) |
|
2:05 - 2:25 p.m. Technical Support for Monitored Natural Attenuation of a Uranium, Thorium, and Mercury Contaminated Wetland along the Savannah River, USA Daniel Kaplan, University of Georgia ► PRESENTATION PDF |
The TNX Area (or T-Area) on the Savannah River Site, South Carolina is a former pilot-scale nuclear facility that released processed waste, including uranium (U), thorium (Th), and mercury (Hg), into a seepage basin adjacent to a wetland between 1958 and 1980. A sequential extraction protocol was used to determine desorption Kd values. Th-Kd values ranged from 115 to 2255 mL/g. U-Kd values ranged from 170 to 6493 mL/g, while Hg-Kd values ranged from 4704 to 5582 mL/g. Compared to (ad)sorption Kd values, these desorption Kd values were appreciably greater because they captured the “aging” process of the radionuclides with the sediment, making the radionuclides more refractory. Compared to nonsite-specific data, these in situ Kd values improved accuracy, were more defensible, and removed unnecessary conservatism for subsequent transport and risk calculations. Additional tests were conducted to provide geochemical information relevant for selecting appropriate remediation technologies for the contaminated site. U LIII-edge XAS spectra of a nearby contaminated wetland indicated that the U in surface sediments, where a vast majority of the contamination existed, was primarily in the U(+6) oxidation state bound to iron-oxides and organic matter. Only in deeper, saturated conditions were U(+4) non-uranite species detected. Based on sequential extraction results, the Hg was strongly associated with iron-oxides and/or some precipitated phases. Only ~8% of the Hg was associated with organic matter. Thermodynamic calculations suggested that meta-cinnabar (HgS) would not be stable in this system due to the relatively low natural pH (pH 4.2), low sulfate concentrations, and high Eh levels. Additionally, U, Th, and Hg were not concentrated with the smaller, clay-sized sediment particles. Given the ecologically sensitive nature of the wetland, the fact that these contaminants were strongly bound to the sediment, and that there was little risk for the contaminated particles to be transported off the site, it was concluded that monitored natural attenuation could be effectively used to complement engineered solutions for lowering human risk. Coauthors: Peng Lin (University of Georgia), Edward J. O’Loughlin (Argonne National Laboratory), Maxim I. Boyanov (Argonne National Laboratory, Bulgarian Academy of Sciences), Kenneth M. Kemner (Argonne National Laboratory) |
| 2:25.- 2:45 p.m. |
Open Discussion __________________________________________________ |
| 2:45 - 3:15 p.m. |
Posters and Vendor Exhibit __________________________________________________ |
|
3:15 - 3:35 p.m. One Resin to Rule Them All: Hybrid Resins Simultaneously Remove Multiple Contaminants from Groundwater Jacqueline Hager, Pacific Northwest National Laboratory ► PRESENTATION PDF |
Pump-and-treat (P&T) facilities are commonly deployed at sites to remediate contaminated groundwater (GW) plumes. Contaminants in GW are removed in aboveground P&T facilities by single or multiple treatment technologies before treated GW is discharged back into the subsurface. Ion exchange (IX) is one technology used to remove aqueous contaminants. IX resins have a versatile design that uses different copolymer backbones and functionalized groups to selectively capture GW contaminants. However, sites with multiple, overlapping contaminant GW plumes may require multiple IX resins to treat each contaminant-of-concern (COC), depending on their speciation. This is the case for the Hanford Site’s 200 West (200W) P&T facility that currently uses two different IX resins to treat uranium (U) and technetium (Tc). Treatment of additional COCs, including iodine-129 (I) and hexavalent chromium (Cr(VI)), is also anticipated with expansion of 200W P&T operations. “Hybrid” resins, which can remove more than one contaminant using IX in combination with additional removal mechanisms (e.g., sorption and redox reactions), offer the potential to remove multiple COCs without necessitating costly P&T infrastructure expansion. In this study, the performance of five hybrid resins was assessed alongside the IX resins currently used at the 200W P&T for the removal of U, Tc, I, and Cr(VI) from a 200W synthetic groundwater. Laboratory-scale batch and column tests were conducted to determine removal mechanisms and kinetics for each resin. Solutions from these tests were analyzed to: (i) compare COC removal performance; (ii) identify potential kinetic limitations; and (iii) evaluate the impact of competitive ions on removal performance. The post-reacted resin beads were also analyzed using a suite of solid-phase characterization techniques to determine the removal mechanisms. These results will inform the use of hybrid resins to treat complex and ever-changing GW conditions, while being easily incorporated into pre-existing P&T infrastructure. Coauthors: Sarah A. Saslow (Pacific Northwest National Laboratory), Elsa A. Cordova (Pacific Northwest National Laboratory), Carolyn I. Pearce (Pacific Northwest National Laboratory), Tatiana G. Levitskaia (Pacific Northwest National Laboratory), Nancy M. Escobedo (Pacific Northwest National Laboratory), Christian D. Johnson (Pacific Northwest National Laboratory), Yilin Fang(Pacific Northwest National Laboratory), Daria Boglaienko (Pacific Northwest National Laboratory), Mark E. Bowden (Pacific Northwest National Laboratory), Nabajit Lahiri (Pacific Northwest National Laboratory), Mark Engelhard (Pacific Northwest National Laboratory), Emily Nienhuis (Pacific Northwest National Laboratory), Jose Marcial (Pacific Northwest National Laboratory) Larry Gottlieb (ResinTech, Inc.) Mark Carlson (Oregon State University), Rob Mackley (Pacific Northwest National Laboratory) |
|
3:35 - 3:55 p.m. The Chemistry of Ra-226 and Other Contaminants in a Historically Contaminated River Bank: Lessons Learned on Kd and the Importance to Check Potential Precipitation Reactions. Nathalie Impens, Belgian Nuclear Research Centre ► PRESENTATION PDF |
The “Sigma plan” https://www.sigmaplan.be/en/ aims to create in Belgium inundation zones along the Grote Nete river to prevent Antwerp from flooding in extreme weather conditions. The riverbanks of the Grote Nete are at some hotspots historically contaminated by the phosphate industry resulting in Naturally Occurring Radionuclides (NOR) legacy. Ra-226 is from a radiation protection point of view one of the most important radionuclides present at the hot spot under study, with a local soil activity concentration higher than 3000 Bq/kg Ra-226. Coauthors: Karl Andreas Jensen (Centre for Environmental Radioactivity), Lindis Skipperud (Centre for Environmental Radioactivity), Axel Van Gompel (Belgian Nuclear Research Centre), Nahtalie Vanhoudt (Belgian Nuclear Research Centre) |
|
3:55 - 4:15 p.m. Influence of Co-Contaminants on the Effectiveness of Ammonia Gas Treatment of Uranium Contamination in Vadose Zone Sediments Jim Szecsody, Pacific Northwest National Laboratory ► PRESENTATION PDF |
The use of a reactive gas, ammonia, for remediation of uranium in vadose zone sediments at the U.S. Department of Energy’s Hanford Site (Richland, WA) has a high potential to decrease mobility of uranium without adding additional water within the vadose zone. Surface and subsurface releases of uranium at the Hanford Site was studied with co-contaminants, depending on the potential waste and chemical process used historically, that included: a) acids (nitric, sulfuric), b) bases, and c) inorganic complexants. Uranium is present as aqueous and adsorbed Ca-uranyl carbonate complexes (groundwater is Ca, Mg-carbonate saturated), carbonate precipitates (e.g., liebigite), and hydrous silicates (e.g., uranophane and Na-boltwoodite). Previous laboratory studies have shown that ammonia treatment of low water content sediments results in dissolution of multiple mineral phases including montmorillonite, muscovite, illite, and kaolinite, and as the pH neutralizes, secondary precipitates form including chrysotile, diaspore, and hematite. Ammonia treatment of U-contaminated sediments from 12 different Hanford disposal sites showed that in most cases (85%) aqueous and adsorbed uranium mobility decreased 60 to 90%, which may be caused by (1) precipitation of uranium minerals or (2) coating of U precipitates by non-U minerals. The focus of this study was to understand the relation between the presence or absence of specific phases or co-contaminants and the change in uranium mobility from ammonia treatment. For example, the presence of high nitrate or sulfate at some waste sites did not influence uranium sequestration using ammonia. However, in one previous study the absence of aqueous carbonate resulted in considerably less decrease in uranium mobility upon ammonia treatment. At one Hanford study disposal site, vadose zone sediments showed higher U leaching with ammonia treatment than no treatment. It is hypothesized that the lack of carbonate in sediments at this acidic disposal site resulted in favorability of other solid uranium phases due to decreasing pH impacting speciation. Coauthors: Michael J. Truex (Pacific Northwest National Laboratory), Hilary P. Emerson (Pacific Northwest National Laboratory), Nikolla P. Qafoku (Pacific Northwest National Laboratory), Jonathan N. Thomle (Pacific Northwest National Laboratory), Lirong Zhong (Pacific Northwest National Laboratory), Silvina A. Di Pietro (Lawrence Livermore National Laboratory) |
|
4:15 - 4:35 p.m. Formation of Mineralogical Interfaces as Radionuclide Repositories Grant Douglas, Commonwealth Scientific Industrial Research Organisation ► PRESENTATION PDF |
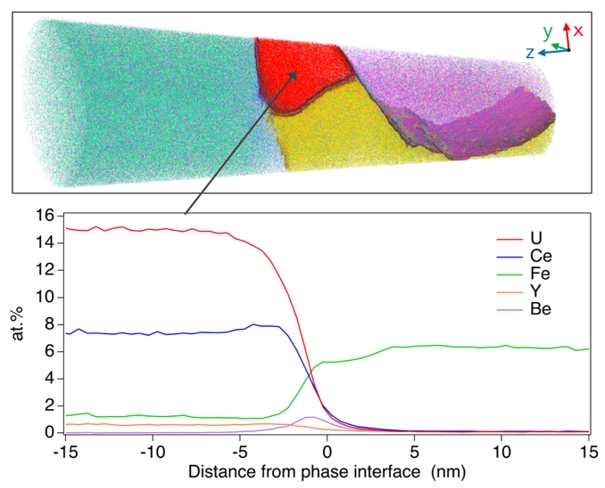 Efficient capture of fugitive actinides and other radionuclides constitutes a pervasive remediation challenge at legacy or nuclear accident sites globally. Hydrotalcites (HTC), a class of layered double-hydroxide, anionic clay minerals are able to contemporaneously sequester a range of radionuclides and other contaminants from solution, hence offering a unique decontamination remedy. HTC, however, do not provide a robust, long-term repository for radionuclide/actinide isolation. We formed HTC via in-situ precipitation in a uranium mine barren lixiviant and thermally transformed the resulting radionuclide-laden, nanoscale HTC. Forensic examination of the amorphized/recrystallised product using Atom Probe Tomography reveals U segregation within nanometre-wide mineral interfaces and local formation of interface-hosted mineral grains. This U-rich phase is also enriched in rare earth elements, a geochemical analogue of many actinides and represents a previously unreported radionuclide interfacial segregation. These U-rich phases associated with the mineral interfaces record a U concentration factor of ~ 50K relative to the original solute demonstrating high extraction and concentration efficiencies. The co-existing host mineral suite of periclase, spinel-, and olivine-group minerals equates to a lower mantle, high P–T mineral assemblage with geochemical and geotechnical properties suitable for disposal in a nuclear waste repository. This study documents the efficient sequestration of radionuclides from contaminated water with this novel, broad-spectrum, nanoscale HTC capture and concentration process constituting a rapid solute decontamination pathway and solids containment option in perpetuity. Coauthors: David Saxey (Curtin University), Steven Reddy (Curtin University) |
| 4:35 - 5:00 p.m. |
Open Discussion and Closing Remarks __________________________________________________ |