Catalysis
Catalysis
Addressing grand challenges
for the future of energy
Addressing grand challenges
for the future of energy
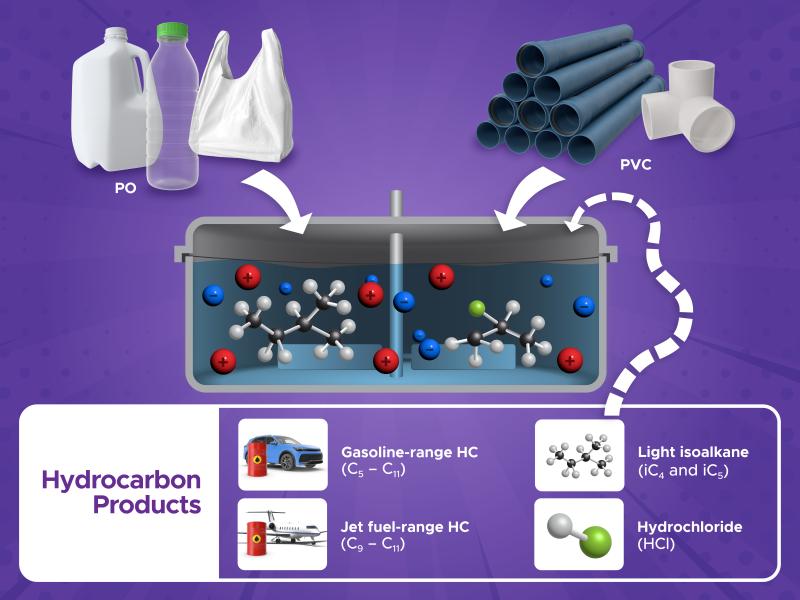
Researchers explore new forms of industrial catalysts used to manufacture raw materials for agriculture and energy production.
Andrea Starr | Pacific Northwest National Laboratory
Our researchers address some of the grandest challenges in catalysis science, including use-inspired design, modeling and simulation, and cost-saving reaction pathways.
New principles of use-inspired design and synthesis will accelerate the development of catalysts that have increased rates for target reactions. New approaches to modeling and simulation, characterization, and synthesis will allow precise control of multidimensional catalytic structures. And finding new reaction pathways will target abundant electrons to convert inexpensive substrates.
The nation has a growing need for energy and pressing needs for secure and resilient fuels. These needs require new chemical transformations that are fast and selective as well as new chemistries and materials for advanced energy storage. The Department of Energy (DOE) recognizes these challenges, and on its behalf, PNNL addresses them.
Research Centers and Facilities
PNNL has a strong foundation of discovery science in catalysis as demonstrated by our extensive research aligned with the DOE Office of Science, Basic Energy Sciences, Catalysis Science Program. This research has resulted in fundamental expertise in catalysis, including through the Center for Molecular Electrocatalysis, which ran from 2009 to 2024.
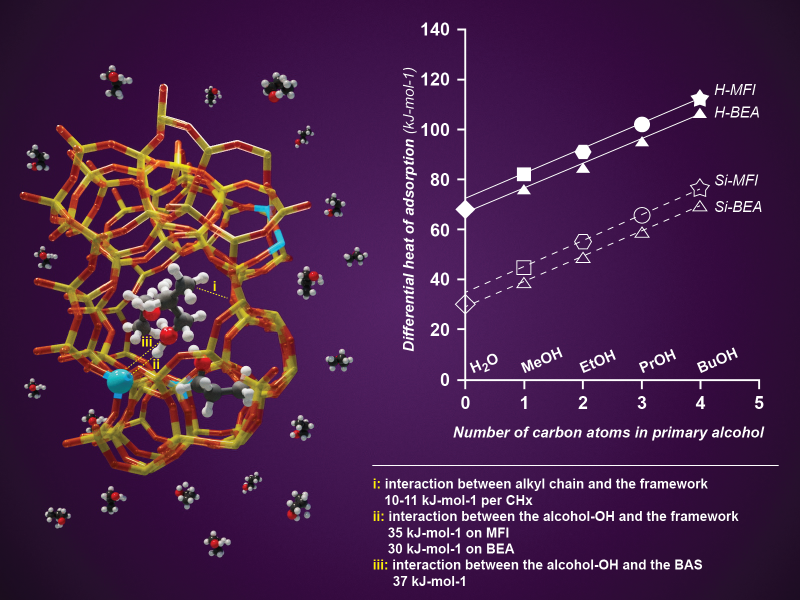
Researchers at PNNL use powerful tools for characterizing, understanding, modeling, and manipulating matter at scales ranging from the atomic to the macroscopic. For additional tools and opportunities, researchers collaborate with colleagues at the Environmental Molecular Sciences Laboratory (EMSL), a DOE user facility located on the PNNL-Richland campus. EMSL houses unique, specialized, and powerful instruments to conduct modeling, simulation, and characterization research.
Through the Institute for Integrated Catalysis (IIC), PNNL combines more than 120 in-house scientists and engineers with experts from universities, industry, and other national laboratories. IIC researchers collaboratively explore and develop the chemistry and technology of catalytic transformations to enable a secure energy future for our nation. They are focused on adding hydrogen to carbon resources, manipulating carbon–carbon and carbon–heteroatom bonds, improving catalysis for exhaust, and storing electrical energy in the form of bonds. These areas of focus are all critical for effectively using elemental and energy resources.
Strategic Investments
Catalysis is also supported by major in-house investments. A Laboratory objective supports research to harness catalysis to create fuels from cheap, abundant domestic materials. Researchers at PNNL draw on the attributes of both natural and synthetic systems to develop catalysts that work at energy-saving low temperatures. They also tailor catalysts for safe and cost-effective applications that avoid unwanted byproducts.