Chemical
Physics
Chemical
Physics
Researchers study biological organic materials, such as proteins and intra- and extracellular structures, to better understand the complex chemical language cells and microbes use to communicate and perform fundamental functions relevant to energy and the environment. During study, researchers often need to move atomic-scale samples while keeping them in controlled environments. The Environmental Transfer Hub (ETH) was designed and built at EMSL, the Environmental Molecular Sciences Laboratory, to transfer materials under controlled environments.
Andrea Starr | Pacific Northwest National Laboratory
Interesting things occur in the places where change is happening: where solid ice meets liquid water, where crystals form on a solid surface, and where molecules condense into new phases of matter. These are the places where chemistry meets physics and where much is learned about the fundamental forces that guide and underpin research. Our goal is to understand the underlying chemical physics of complex processes relevant to energy production and use, waste management, and national security.
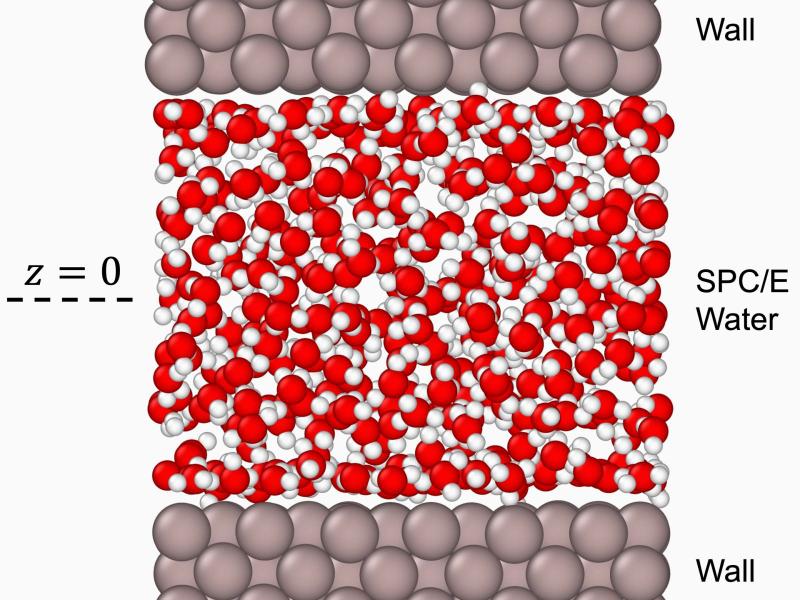
The places where liquid meets solid and where gas condenses to liquid are challenging interfaces to study, yet essential to understand. Liquid/liquid and liquid/solid interfaces built one molecule at a time provide uniform structures whose chemistry can be understood and visualized at a level of detail not available in bulk materials. Similarly, we can isolate individual ions solvated by adding one solvent molecule at a time. We use X-ray spectroscopic methods that span femtoseconds to milliseconds to study how solutions behave under extreme experimental conditions. We combine those results with advanced theory and modeling methods to help interpret the results. We study pattern formation and self-assembly, phase transitions, and crystal nucleation, all in the service of better understanding the fundamental physical properties that underlie nearly every facet of the natural world.
Our approach is to develop the advanced experimental and theoretical tools needed to make highly quantitative experimental measurements of molecular-level processes. These processes lend themselves to rigorous and accurate theoretical modeling and simulation. Our goal is to provide a detailed understanding that can lead to mechanistic descriptions of complex processes, using a combined experimental and theoretical approach. We combine advanced theory, computational modeling, and experiments to explore the molecular world. Investments in chemical imaging technology, computational capabilities, and modeling methods are core to our work.