Core Basic Energy Sciences Catalysis Program
Transdisciplinary Approaches to Realize Novel Catalytic Pathways to Energy Carriers
Basic energy research for catalysis combines chemical synthesis, theory, and imaging to design active catalytic centers and their environments to optimize selective catalytic pathways for renewable fuel production. This cross-disciplinary program integrates bioinspired, homogeneous, and heterogeneous catalysis, as well as surface science.
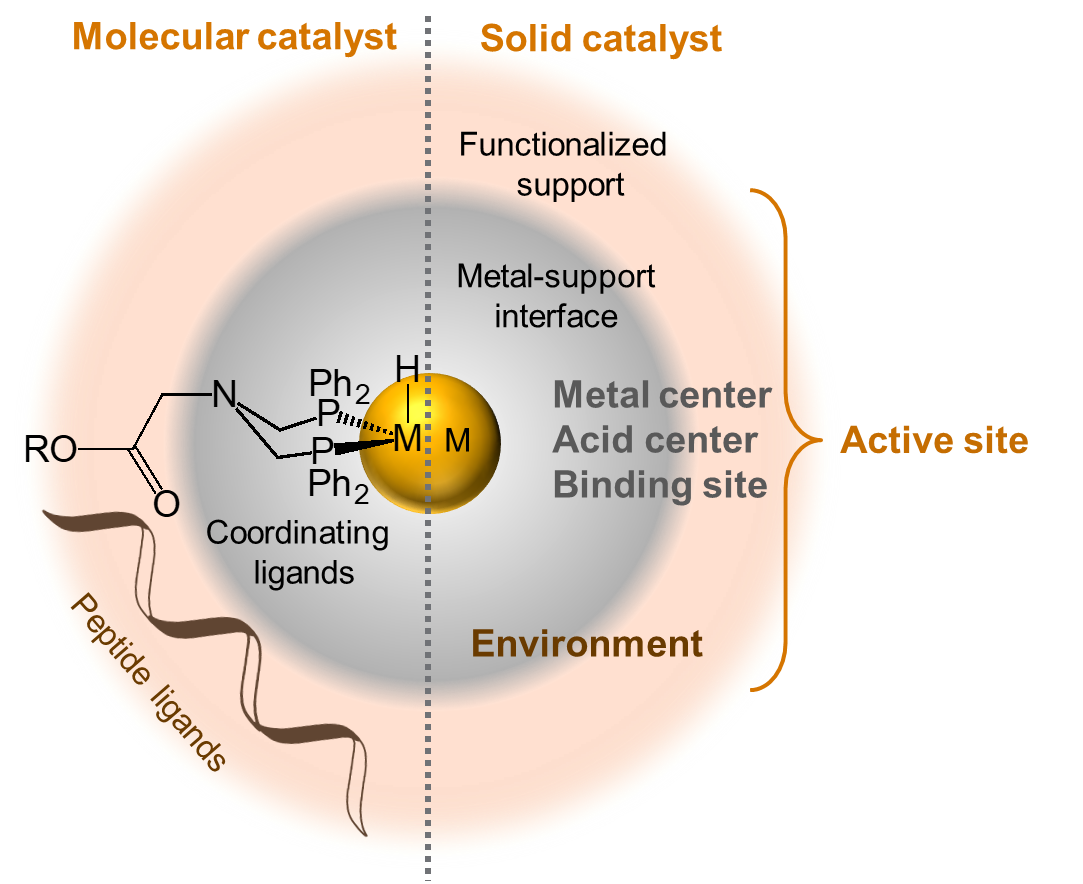
The active center of a catalyst determines its intrinsic reactivity and the specific chemical reaction it assists. The catalytic environment helps selectively guide and enhance a reaction. High reaction rates under mild conditions, coupled with high selectivity, are key attributes needed for more flexible production of fuels and chemicals.
In this program, researchers aim to catalyze carbon–carbon and carbon–hydrogen bond formation, as well as carbon–oxygen bond cleavage, on acid–base and hydrogenation sites with high activity and selectivity. Scientists hypothesize that selected pathways for these reactions can be markedly enhanced by:
- positioning functional groups around the active center to stabilize reactants during the reaction
- adjusting the available space around the active center to match the shape of the rate-limiting transition state
- encouraging self-organization of solvent and reactant molecules around the active center.
Thrust 1: Tailoring acid–base catalysis for elimination and C-C bond formation
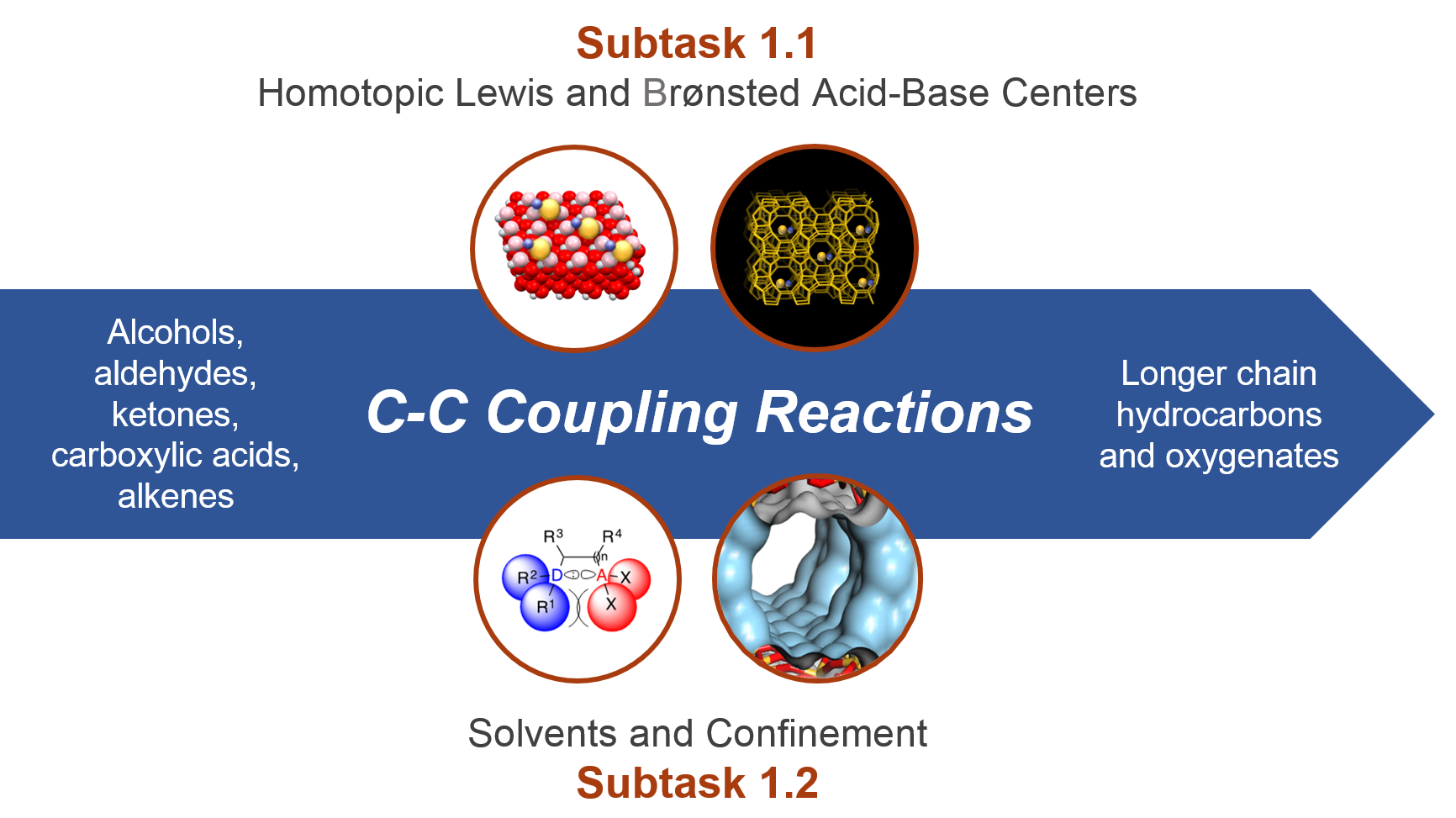
This thrust explores how the geometric and electronic properties of Lewis and Brønsted acid–base catalytic sites affect carbon–carbon bond formation. Researchers first synthesize precise metal or metal-oxide active sites. Then they stabilize those sites in zeolites, faceted oxides, inert substrates, mesoporous solids, and homogeneous catalysts.
Spectroscopic characterization of active sites and the associated molecular transformations provides experimental data to couple with theoretical investigations. From this information, researchers produce detailed, molecular-level understanding of the factors that control function at these active centers.
Thrust 2: Enhancing H2 addition rates by designing the metal center
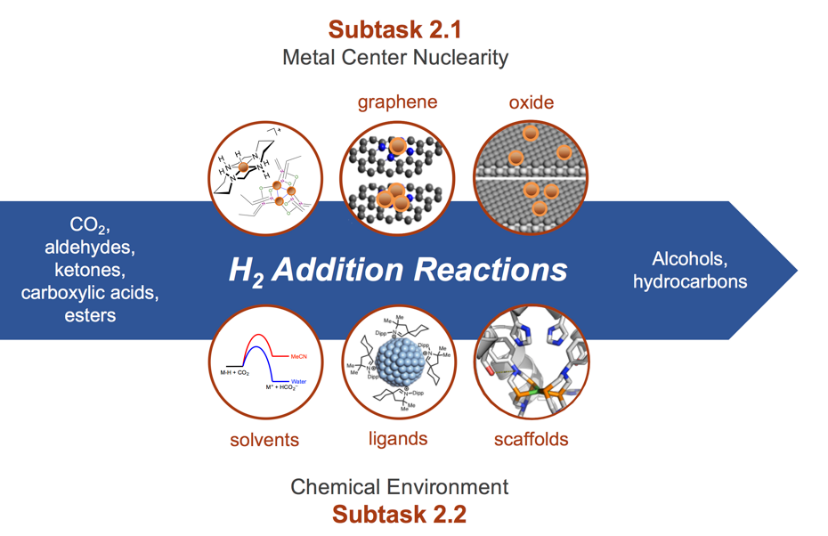
This thrust addresses the fundamental principles of hydrogen addition to carbon–oxygen bonds in CO2, alcohols, aldehydes, ketones, and carboxylic acids, as well as carbon–carbon bonds in aromatic alcohols or ethers.
For these reduction reactions to run at lower temperatures in industrial processes, engineers need catalysts with higher activity. In this thrust, researchers study novel, highly active sites of single metal atoms or small defined clusters on scaffolds that include organic ligands, enzyme-inspired outer coordination sphere, graphene, zeolites, or microporous oxides. They also study how solvents, confining supports, organic ligands, or a bioinspired scaffold influence reaction rates and mechanisms. This work involves a multidisciplinary team with expertise in theory, surface science, and homogeneous and heterogenous catalysis.
Crosscutting Theory Subtask: Modeling active centers, environments, and their roles in catalytic transformations
In this crosscutting subtask, researchers employ theory and computation to develop and refine mechanistic hypotheses; formulate structure–activity relationships; and interpret experimental data. This task utilizes state-of-the-art computational infrastructure and methodologies in modern simulation to formulate and address advanced hypotheses.
Researchers in this subtask pursue two scientific objectives: (1) quantitative energy landscapes of complex catalyst systems and (2) rigorous description of dynamics and collective motions of catalytic sites and their environment. Through these objectives, researchers in the institute better understand reactivity at the complex interfaces found in Thrusts 1 and 2.
- Principal Investigator (PI): Johannes Lercher
- Subtask PIs: Aaron Appel, Zdenek Dohnálek, Roger Rousseau, and Janos Szanyi
- Co-PIs and Key Personnel: S. Thomas Autrey, R. Morris Bullock, David A. Dixon (University of Alabama), Enrique Iglesia (University of California, Berkeley), John L. Fulton, Feng Gao, Bojana Ginovska, Vassiliki-Alexandra Glezakou, Oliver Y. Gutiérrez, Jian Zhi Hu, Enrique Iglesia (University of California, Berkeley), Andreas Jentys (Technical University of Munich), Abhijeet Karkamkar, Bruce D. Kay, Greg A. Kimmel, Libor Kovarik, Joseph A. Laureanti, Mal-Soon Lee, John C. Linehan, Nikolay G. Petrik, Gregory K. Schenter, Wendy J. Shaw, Huamin Wang, Yong Wang (Washington State University), and Eric S. Wiedner