Nanoscale Analysis Provides Key Answers for Modeling Mineralization in Basalt
PNNL research unlocks key findings needed for potential commercial deployment of carbon mineralization technology
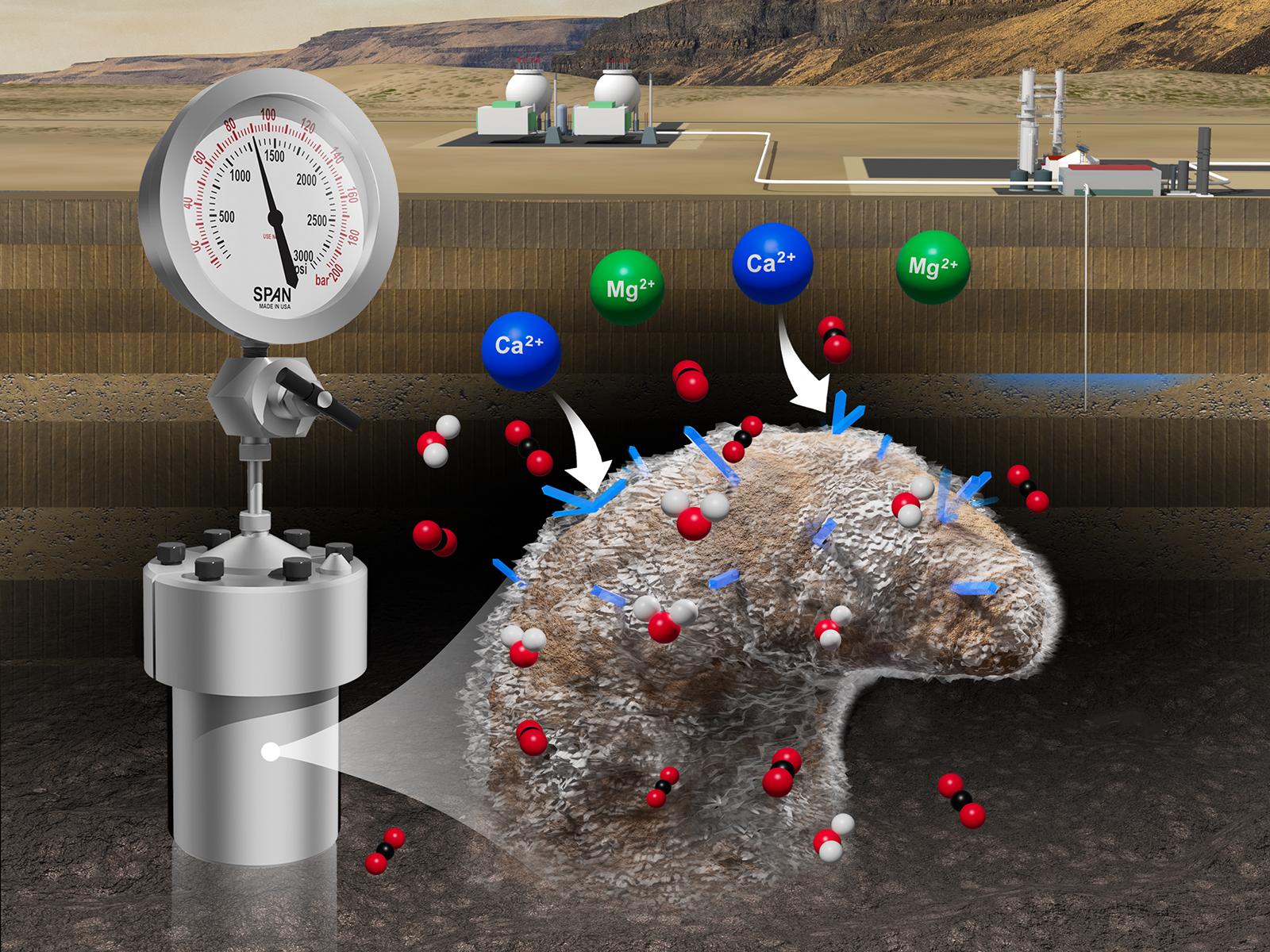
A methodology on resolving nanoscale processes during carbon mineralization—discovered and led by Pacific Northwest National Laboratory Post Doctoral Researcher Xiaoxu Li and Chemist Emily Nienhuis—provides insight into the previous knowledge gaps needed for accurate reservoir models. Their research was recently published on the cover of Environmental Science & Technology Letters, a journal of the American Chemical Society.
(Composite image by Mike Perkins | Pacific Northwest National Laboratory)
Removing carbon dioxide (CO2) from industrial emissions and from the atmosphere and then safely storing it into the Earth’s deep subsurface is becoming increasingly essential to meeting decarbonization goals and preserving a livable planet.
Pacific Northwest National Laboratory (PNNL) scientists discovered how to store supercritical CO2—carbon dioxide in its fluid state—in basalt reservoirs safely and permanently. This process is called geologic carbon sequestration, or carbon mineralization. But for the technology to be deployed commercially in the United States a Class VI well permit must first be attained.
“In order to apply for and be issued this permit, there has to be what is called a reservoir model for us to understand the fate and behavior of the injected CO2,” said PNNL Chemist Emily Nienhuis. “In other words, if we inject x amount of CO2 into a reservoir, where does it go? And how long does it take to mineralize or become rock?”
However, current reservoir models do not account for how water-bearing supercritical CO2 reacts with the minerals in basalt. Doing so requires reaction rates for each individual mineral species with water-bearing supercritical CO2 under reservoir conditions.
A methodology discovered and led by PNNL postdoctoral researcher, Xiaoxu Li, and Nienhuis provides insight into the previous knowledge gaps needed for accurate reservoir models. Their research was recently published in Environmental Science & Technology Letters, a journal of the American Chemical Society.
“Modeling subsurface processes requires us to look at what is happening at the nanoscale level, but up to now there has been no technology to do that,” said Li. “So, we made a trick.”
The “trick” is a new method, called Identical Location-Transmission Electron Microscopy (IL-TEM). The transmission electron microscope is in EMSL, the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility at PNNL. It provides highly magnified images to demonstrate individual mineral reactivity after exposure to CO2 fluids at reservoir conditions and provides information on kinetics and mineralization behavior at the nanoscale.
“We nicknamed it ‘Freeze and Look,’” said Li, “because we freeze the reaction under the liquid nitrogen temperature for characterization then simulate the underground reaction with high temperature and high pressure.”
The sample is then taken out of the reactor to identify how the structure of the sample changed, including elemental distribution and the underlying mechanisms of reaction at the single particle level.
“It is exciting to observe a similar phenomenon to what happed in the field at the Wallula Basalt Sequestration Pilot,” said Li.
Using IL-TEM, the research team saw aragonite nanocrystals, which was also one of the species seen at the larger-scale laboratory studies and in the field test site for the PNNL-led Wallula Basalt Sequestration Pilot Project, where the first discovery of subsurface carbon mineralization in basalt occurred.
“These experimental techniques are typically seen in the fundamental science space but we’re applying them to more complex problems in an applied space, which is a very unique aspect of this work,” said Nienhuis. “We’ve got these very aggressive carbon negative goals in the future and it's going take a lot of people, a lot of different techniques, and a lot of solutions to try and figure out how to achieve that.”
To date, no Class VI well permits have been issued for injection of CO2 in basalt reservoirs. IL-TEM provides missing key information to enhance model subsurface processes and help make the process for a Class VI well permit more attainable—a big milestone for future deployment of commercial carbon mineralization technology.
“Throughout Earth's geological history, we have witnessed five major mass extinction events and, now, it appears we might be experiencing a sixth,” said Li. “I am convinced that we stand at a pivotal juncture. Without significant and immediate actions to improve our environmental stewardship, we risk plunging into a scenario reminiscent of past biological extinctions. It is exciting that we have a solution using basalt to help the planet recover.”
Published: December 21, 2023