Identifying the Active Species that Catalyze Low-Temperature Polyolefin Upcycling
The low-temperature breakdown of polyolefins is driven by carbenium ions generated from chloroaluminate ionic liquids
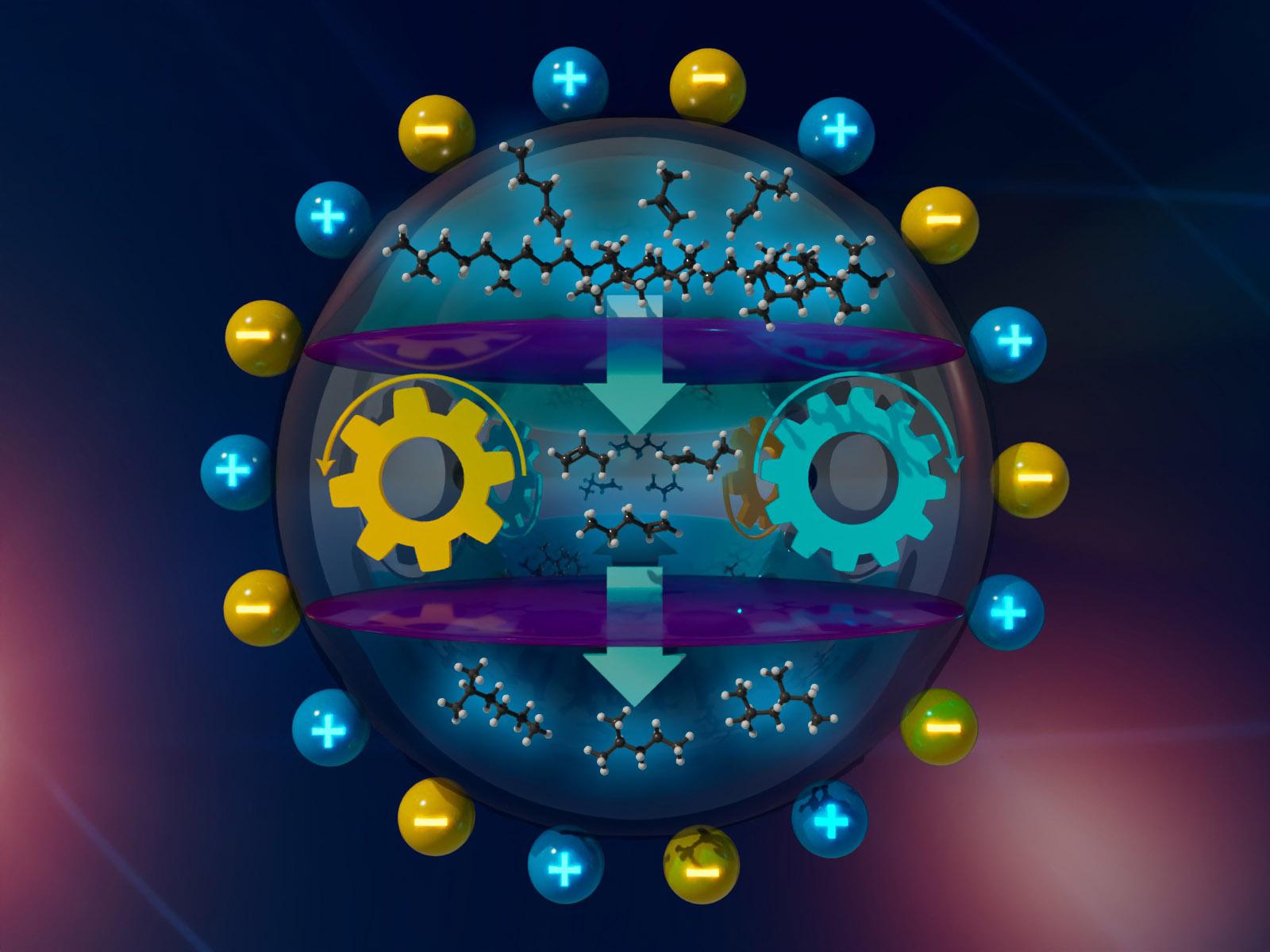
Tandem cracking-alkylation with chloroaluminate ionic liquids enables low-temperature conversion of polyolefins into liquid alkanes.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Chloroaluminate ionic liquids can selectively convert discarded polyolefins into gasoline-range alkanes at temperatures below 100 °C. However, questions have been raised about the speciation of chloroaluminate and its role in the polyolefin deconstruction process. Combined in situ and operando spectroscopies elucidate the nature and structural dynamics of chloroaluminate species within ionic liquids. Polyolefin deconstruction begins with small amounts of a chloride additive as a carbenium ion initiator, reacting with an in situ generated aluminum chloride monomer, providing initial carbenium ions. The resulting carbenium ions activate the polymer strands and advance the cracking-alkylation process through multiple hydride transfer reactions. Alkenes that form during cracking rapidly enter the alkylation cycle.
The Impact
Upcycling polyolefin wastes into products compatible with existing markets is crucial to effectively managing resources. Polyolefin waste could be a promising feedstock for next-generation refineries, with volumes (~160 Mt/yr-1; 2019) equivalent to 1 billion barrels of crude oil. However, current technologies to break down spent polyolefins use catalytic pyrolysis and hydrocracking over solid acid catalysts that require significant energy input at high temperatures (T > 300 °C). By understanding the nature and performance of the catalytically active species in low-temperature polyolefin deconstruction, scientists can effectively design a new generation of catalysts. These catalysts will enable the reliable upcycling of waste polyolefins with low energy consumption.
Summary
The low-temperature kinetically coupled cracking and alkylation of polyolefins with iso-paraffins involves two active species of chloroaluminate ionic liquids. The initial main chloroaluminate ion species are the monomer, generated in situ through the dissociation of Al2Cl7−, and its reactive adduct with tert-butyl chloride. The adduct undergoes reversible transformation into a tert-butyl carbenium ion and AlCl4− ion pair. Subsequently, hydride transfer from the formed tert-butyl carbenium ion leads to carbenium ions in the alkane polymer strands and alkylating agent. Hydride transfer is rapid and facilitated by the high ionic strength of the environment. The propagation of carbenium ion intermediates by hydride transfer always involves species that are associated and stabilized by AlCl4‒ anions. Computational results strongly support these conclusions. Additional olefin exhibits a preference for oligomerization over alkylation, as confirmed by operando infrared studies and kinetic analyses that indicate concurrent cracking and alkylation. Carbenium ions in the alkane polymer strands isomerize and undergo cleavage by β-scission, yielding shorter carbenium ions and alkenes. The alkenes actively participate in the alkylation cycle, rapidly adding to carbenium ions from isopentane. This mechanistic understanding will propel the advancement of catalytic polyolefin transformation and assist in developing a next-generation catalyst family for polyolefin deconstruction.
Contact
Sungmin Kim, Pacific Northwest National Laboratory, sungmin.kim@pnnl.gov
Funding
We thank Camelia N. Borca from the Phoenix beamline at the Swiss Light Source (SLS) of the Paul Scherrer Institute in Switzerland for their assistance in Al X-ray absorption spectroscopy characterization. We also thank G.L. Haller (Yale University), J.G. Chen (Columbia University), S.L. Scott (University of California Santa Barbara), and M.L. Sarazen (Princeton University) for their discussion of the manuscript and helpful suggestions. J.A.L., W.Z., S.K., L.H., W.H., J.M., B.Y., O.Y.G., D.R., J.F., D.M.C., J.H., H.W., and M.L. thank the Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences and Biosciences (towards a polyolefin-based refinery: understanding and controlling the critical reaction steps, FWP 78459) for funding support. J.H. also thanks the Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences and Biosciences (Multifunctional Catalysis to Synthesize and Utilize Energy Carrier, FWP 47319) for funding support of in situ nuclear magnetic resonance work. Computational work was performed using the National Energy Research Scientific Computing Center located at Lawrence Berkeley National Laboratory provided by a user proposal and the Research Computing Facility at Pacific Northwest National Laboratory.
Published: May 23, 2025
Zhang, W., Khare, R., Kim, M., Hale, L., Hu, W., Yuan, C., Sheng, Y., Zhang, P., Wahl, L., Mai, J., Yang, B., Gutiérrez, O. Y., Ray, D., Fulton, J., Camaioni, D. M., Hu, J., Wang, H., Lee, M.-S., Lercher J. A. 2024. “Active species in chloroaluminate ionic liquids catalyzing low-temperature polyolefin deconstruction,” Nat. Commun. [DOI: 10.1038/s41467-024-49827-4]