Ion Behavior Near Interfaces Affects Aggregation
Increasing concentrations of ions slows the rate of particle aggregation in boehmite suspensions
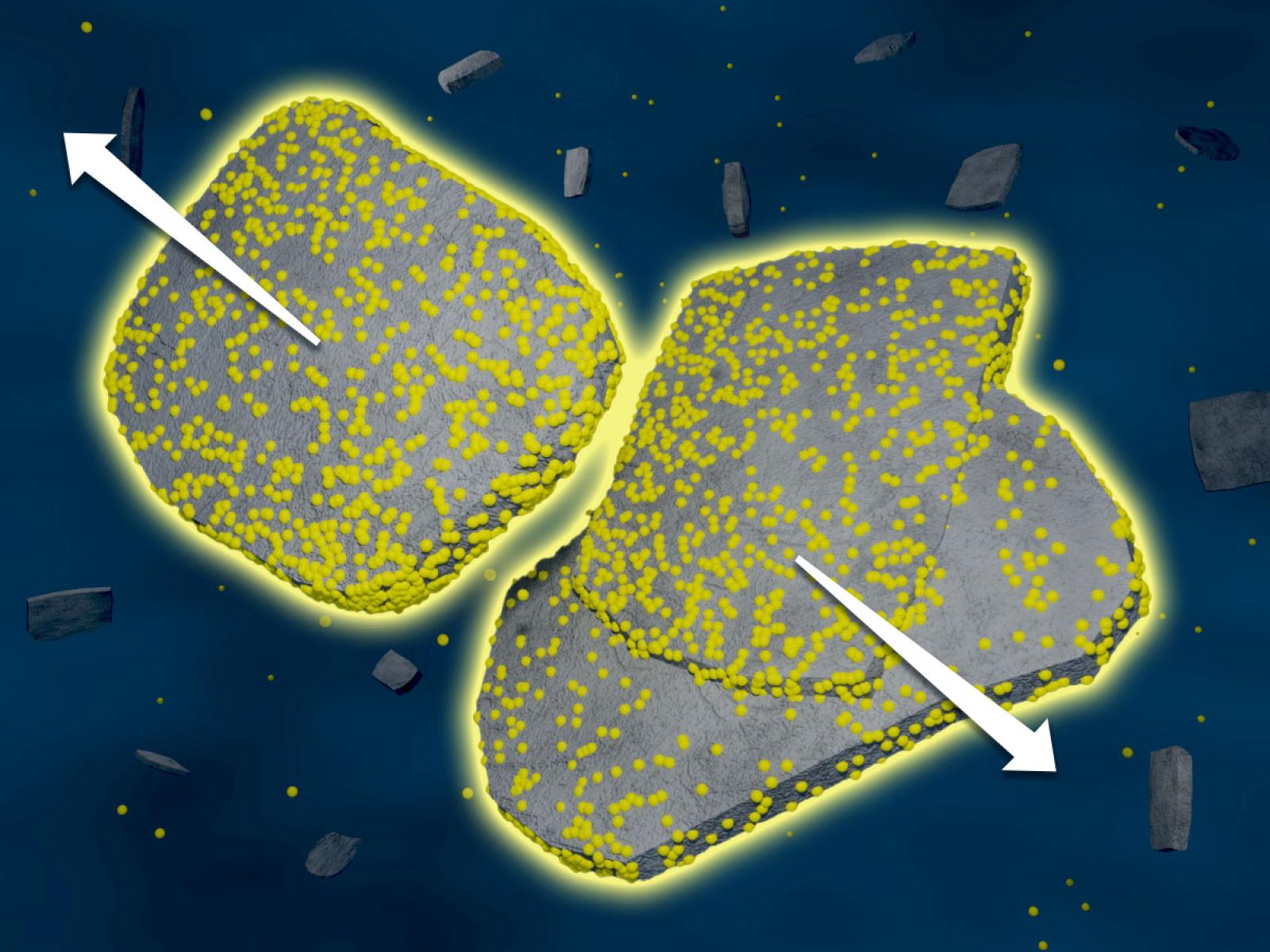
As the concentration of ions in a solution increases, nanoparticles are slower to aggregate, likely due to an increase in ions associated with particle surfaces.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Interactions between ions and water play a critical role in how nanoparticles aggregate, but classical representations of nanoparticle behavior do not fully account for molecular-scale forces. Scientists from the Ion Dynamics in Radioactive Environments and Materials (IDREAM) Energy Frontier Research Center studied how ions affected the aggregation of nanoparticles in water. They found that increasing the concentration of ions in solution led to a decrease in the rate of particle aggregation that could not be explained by classical aggregation theories. Results of experiments and simulations showed a change in the active molecular-level forces that could be related to increased numbers of associated ions.
The Impact
Nanoparticles are central in a wide range of applications, ranging from emerging technologies, to processing of legacy radioactive tank waste at locations such as the Hanford Site. These particles are not static and continuously interact, changing based on the conditions of the solution and their surfaces. Traditional approaches to representing nanoparticle behavior primarily focus on the particles themselves, neglecting the solution and its chemistry. This research highlights the importance of considering solution structure when modeling or trying to understand nanoparticle aggregation, particularly at high ion concentrations.
Summary
The connections between solution structure, particle forces, and system behavior at solid–liquid interfaces remain ambiguous. Researchers used boehmite aggregation, important to understand for legacy radioactive tank waste processing, to establish a connection between interfacial solution structure, emerging hydration forces between two approaching particles, and the resulting structure and kinetics of particle aggregation. They observed a dependence of aggregation rate on ion concentration that does not agree with traditional continuum-based theories that predict an increase in rate with an increase in ionic strength. The measured rate was 15-fold lower in 4 molal solutions than in 1 molal solutions, which is accompanied by an increase in repulsive hydration forces as observed by microscopy. Molecular dynamics simulations indicate that these changes can be attributed to enhanced ion interactions near the interface, resulting in loosely bound aggregates that retain electrolyte between the particles. These insights can facilitate the more accurate prediction of particle aggregation, attachment, and assembly across a range of nanoparticulate systems beyond boehmite.
Contact
Elias Nakouzi, Pacific Northwest National Laboratory, elias.nakouzi@pnnl.gov
Carolyn Pearce, Pacific Northwest National Laboratory, carolyn.pearce@pnnl.gov
Funding
This work was supported by IDREAM (Ion Dynamics in Radioactive Environments and Materials), an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science (SC), Basic Energy Sciences (BES) program, under FWP 68932 at Pacific Northwest National Laboratory (PNNL). PNNL is a multiprogram national laboratory operated for DOE by Battelle Memorial Institute under Contract DE-AC05-76RL0-1830.
Published: May 20, 2025
Butreddy P., J. Heo, N. Rampal, T. Liu, L. Liu, W.T. Smith, X. Zhang, M. P. Prange, B. A. Legg, G. K. Schenter, J. J. De Yoreo, J. Chun, A. G. Stack, and E. Nakouzi. 2024. "Ion correlations decrease particle aggregation rate by increasing hydration forces at interfaces." ACS Nano, 18, 26047–26055 (2024). [doi: 10.1021/acsnano.4c05563]