Enzymatic Control of Carbocation Chemistry
Combined experimental and theoretical study showed how model enzymes control challenging reactions involving highly reactive carbocations
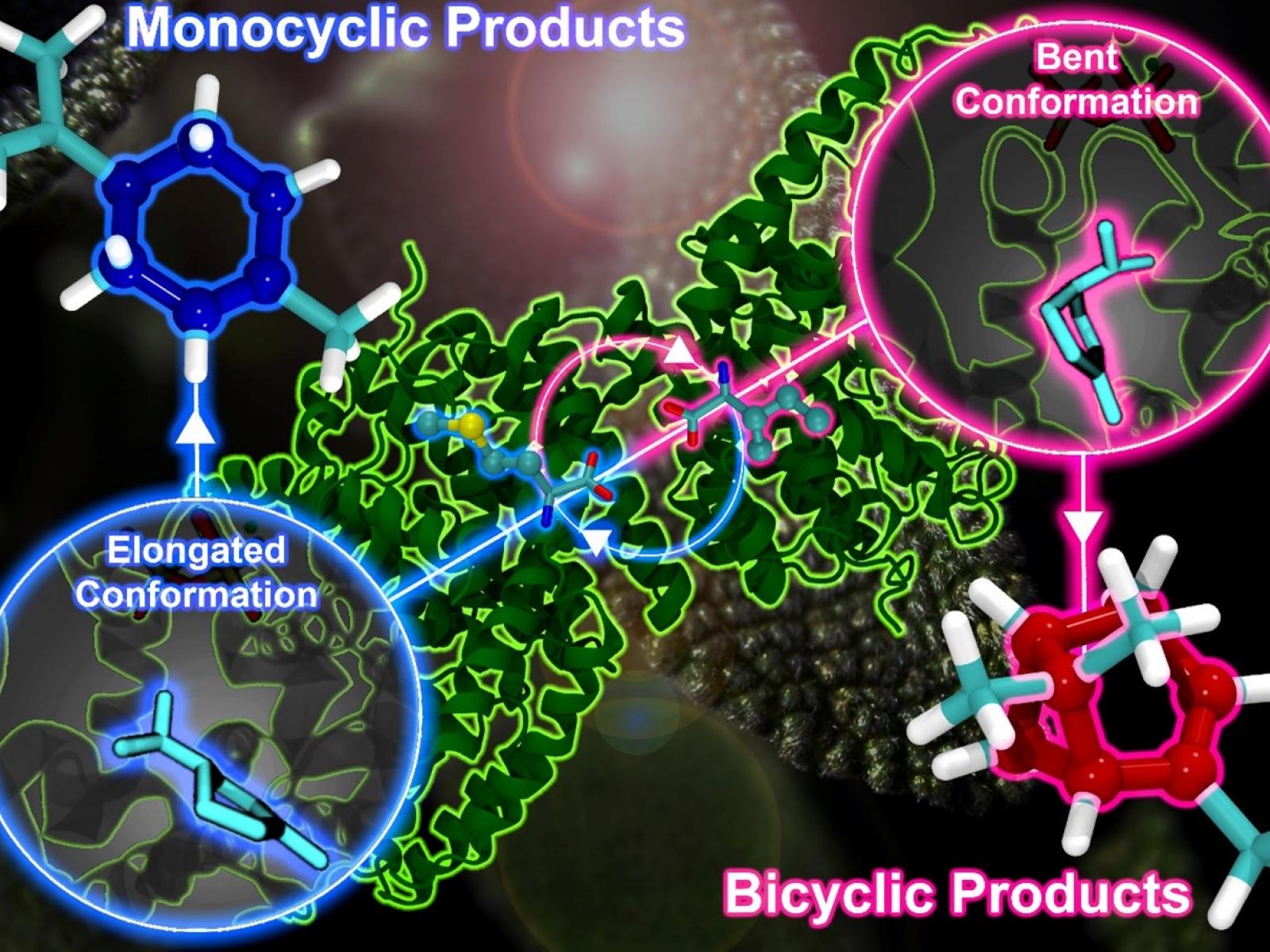
Interactions with parts of the enzyme lead to a preferred conformation of a carbocationic reaction intermediate, which in turn dictates the final reaction product.
(Image by Hoshin Kim | Pacific Northwest National Laboratory)
The Science
Monoterpenes are molecules that play an important role in how plants communicate with other organisms. In nature, their creation is facilitated by a class of enzymes known as monoterpene synthases (MTSs). Using the same starting material, different MTSs generate different monoterpenes by precisely controlling challenging reactions that involve highly reactive carbocations, in some cases with remarkable product specificity and enantioselectivity. Researchers studied the activity of dozens of variants of two model MTSs. Through an integrated series of advanced characterization experiments and atomistic simulations, they found that the shape of a key intermediate determines the final structure of the product.
The Impact
In addition to acting as communication molecules, monoterpenes are important components of essential oils used in the flavor and fragrance industries. They are also under investigation as potential high-energy constituents of aircraft fuels. However, their precise synthesis outside of biological systems can be challenging. This work deepens scientific understanding of how MTSs influence monoterpene formation. More broadly, the results have implications for improving catalytic conversion of highly reactive positively charged organic ions, known as carbocations.
Summary
MTSs catalyze the initial committed step in the biosynthesis of monoterpenes, a challenging reaction involving highly reactive carbocations. MTSs can produce products with remarkable specificity and enantioselectivity. New research used two well-characterized MTSs as models, (4S)-(−)-limonene synthase (LMNS) and (+)-bornyl diphosphate synthase (BPPS), to explore MTS reactivity. Scientists employed an iterative approach that involves comparative atomistic simulations and experimental testing of both the naturally occurring enzymes and 36 additional variants to identify how these enzymes control selectivity. They found that a common reaction intermediate, the α-terpinyl cation (ATC), preferentially adopts one of two different shapes in LMNS and BPPS. This leads to the formation of monocyclic monoterpenes in the former and bicyclic products in the latter. Amino acids within the MTS catalytic pocket interact with the ATC through exquisite steric and electrostatic interactions, thus controlling the reaction outcome. The underlying structures identified in the different MTS models can be broadened to other systems with either confined environments or reactive carbocation intermediates.
This work was performed in part at the Environmental Molecular Sciences Laboratory, a Department of Energy user facility located at Pacific Northwest National Laboratory.
PNNL Contact
Simone Raugei, Pacific Northwest National Laboratory, Simone.Raugei@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences.
Published: August 10, 2022
H. Kim, N. Srividya, I. Lange, E. W. Huchala, B. Ginovska, B.M. Lange, S. Raugei. 2022. “Determinants of Selectivity for the Formation of Monocyclic and Bicyclic Products in Monoterpene Synthases,” ACS Catalysis, 12, 7453. [DOI: 10.1021/acscatal.2c01836]