Cobalt Slows the Low-Water Carbonation of Silicate Mineral Ores
Cobalt substitution for magnesium in a model mafic mineral dramatically slows reaction with carbon dioxide to form a cobalt-doped carbonate
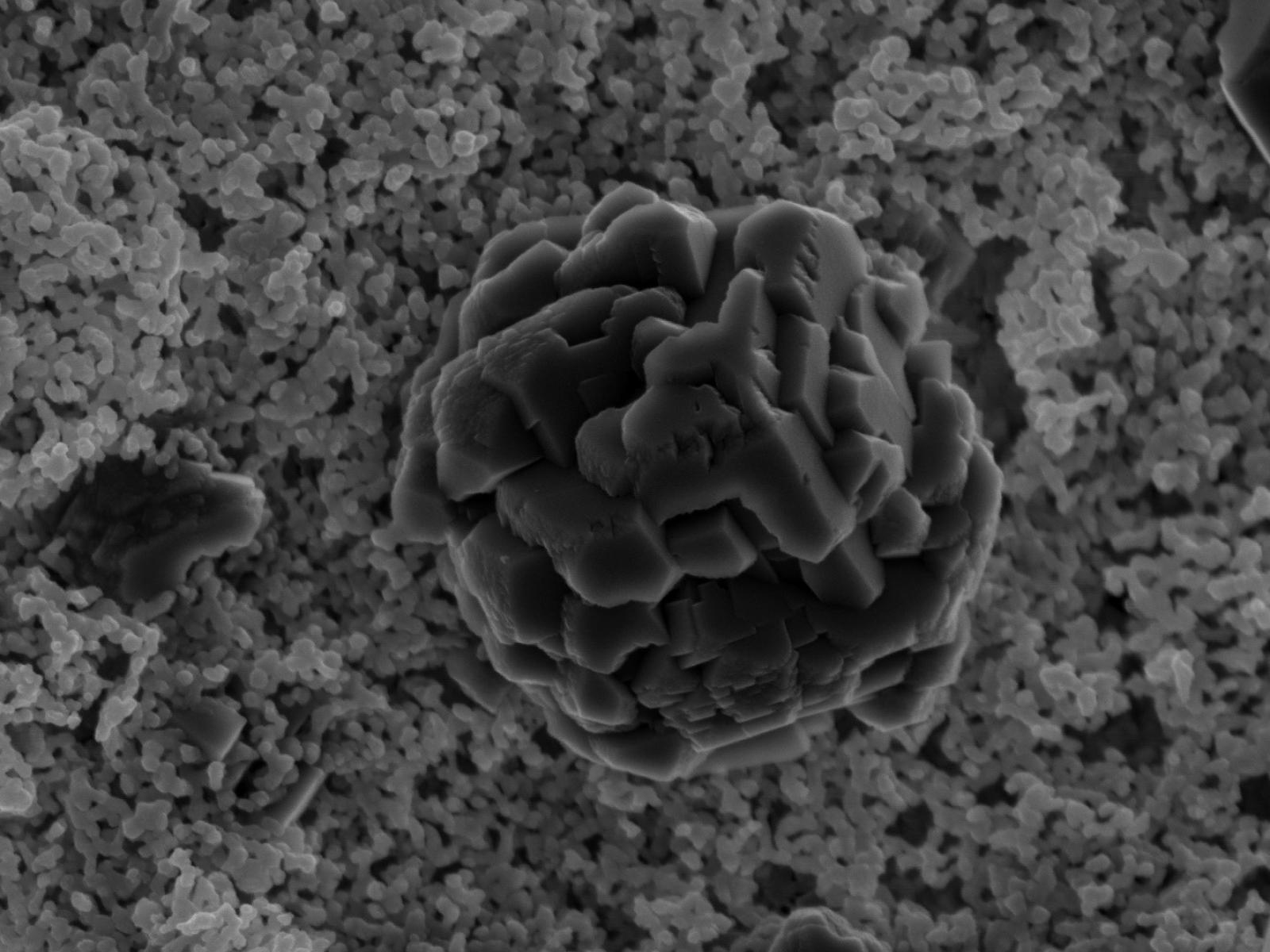
Cobalt-doped magnesite forms more slowly than magnesite in an undoped system, likely due to thermodynamic factors.
(Image by John Loring | Pacific Northwest National Laboratory)
The Science
Demand for cobalt (Co) has surged due to its increased use in metal superalloys and cathodes for rechargeable batteries. Mafic and ultramafic rocks are abundant low-grade Co ores that can react with carbon dioxide (CO2) to form stable carbonate minerals. Researchers investigated the impact and fate of Co during metal silicate carbonation under low water, humidified CO2 conditions. The results show that Co-doped forsterite ((Mg,Co)2SiO4) carbonated to Co-rich magnesite ((Mg,Co)CO3), but the rates of carbonation were an order of magnitude slower than for pure forsterite at similar conditions.
The Impact
Carbonation could improve the economic viability of Co extraction from mafic or ultramafic rocks by lowering processing costs. A low-water approach has the potential to reduce water usage, decrease waste, and favor the most thermodynamically stable carbonate products. This work shows how Co substitution in a model mafic mineral slows carbonation rates and identifies the predominant reaction product. Understanding how Co affects metal silicate carbonation as well as its final fate is beneficial to developing new methods to recover Co from mafic and ultramafic ores.
Summary
High-grade ores of Co are becoming scarce. There is an urgent need to find alternate Co sources to meet the increasing demand for this critical element. Mafic and ultramafic silicate minerals are abundant, yet low-grade, Co ores that can react with CO2 to form carbonate minerals. Researchers used in situ high-pressure infrared spectroscopy to show that Co substitution for Mg in forsterite slows carbonation rates under low-water, humidified CO2 conditions compared to the pure, Co-free mineral. The Co-substituted system gives Co-doped magnesite as the predominant product. The lower reaction rates are likely due to thermodynamic drivers that cause the water films on Co-doped forsterite to be much less oversaturated with ions that precipitate as Co-doped magnesite than in the undoped system. This fundamental knowledge will help researchers develop methods that lower the cost of Co extraction from mafic or ultramafic rocks while decreasing water usage and waste.
Contact
John Loring, Pacific Northwest National Laboratory, john.loring@pnnl.gov
Sebastien Kerisit, Pacific Northwest National Laboratory, sebastien.kerisit@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences program, Chemical and Materials Sciences to Advance Clean Energy Technologies and Low-Carbon Manufacturing (FWP 80281). Pacific Northwest National Laboratory (PNNL) is a multiprogram national laboratory operated for DOE by the Battelle Memorial Institute under Contract DE-AC05-76RL01830. Part of this research was performed at EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at PNNL sponsored by DOE’s Biological and Environmental Research program and located at PNNL in Richland, WA.
Published: January 9, 2025
J.S. Loring, T.E. Webb, M.E. Bowden, M.H. Engelhard, and S.N. Kerisit. 2024. “Cobalt substitution slows forsterite carbonation in low-water supercritical carbon dioxide,” Physical Chemistry Chemical Physics 26, 26465-26471. [DOI: 10.1039/d4cp02092h]