Cation Effects on Radicals from Nitrite
The size of cations in highly concentrated nitrite solutions affects the production of radicals
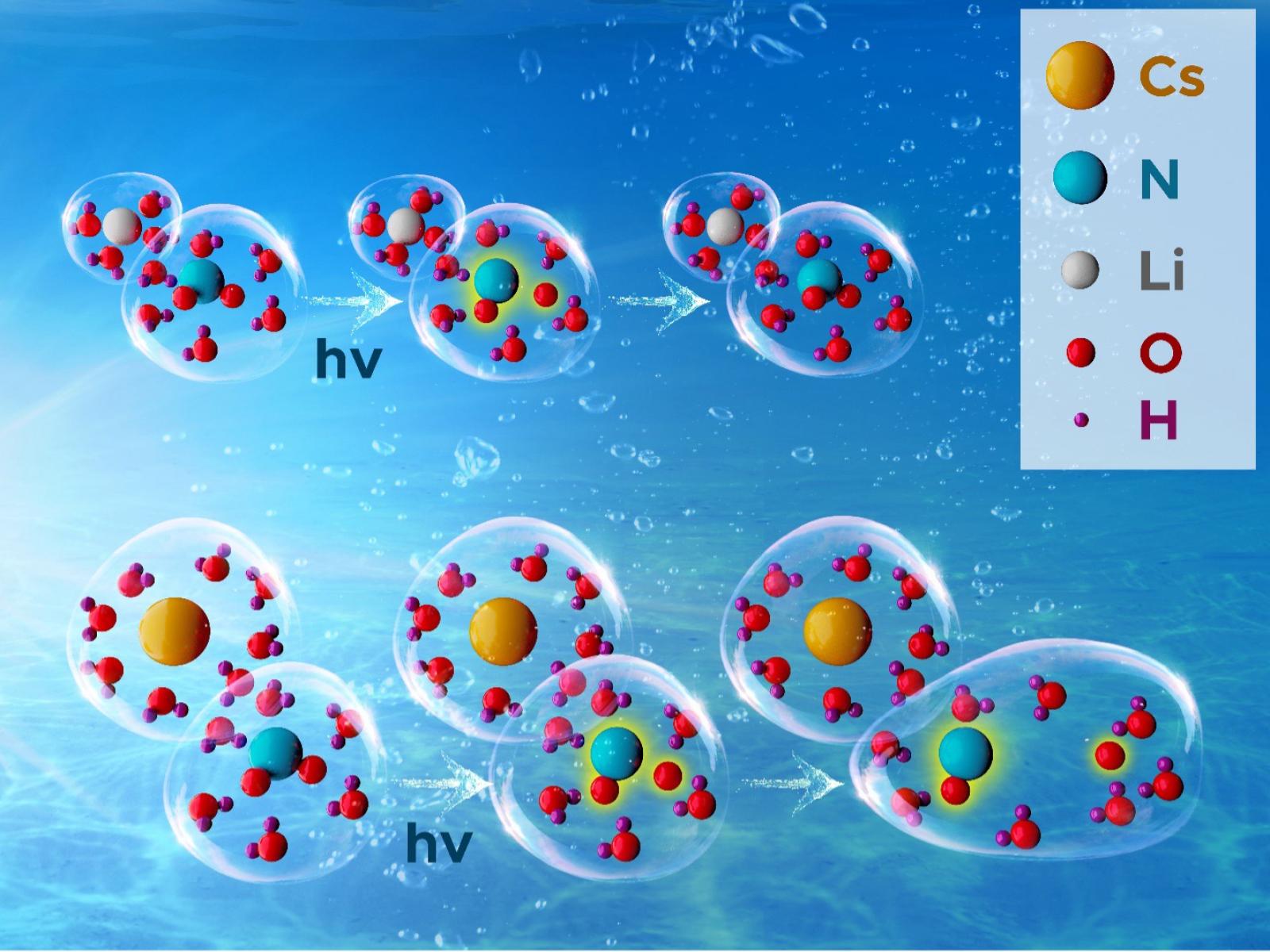
In highly concentrated and alkaline nitrite solutions, radical formation is controlled by cation effects on the overall structure and organization of the solution.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Radicals can be formed by various types of radiation, from ultraviolet light to the ionizing radiation present in legacy tank waste. Nitrite ions, when exposed to radiation, form a range of highly reactive and short-lived radicals. Researchers explored the formation of radicals in concentrated and alkaline nitrite solutions exposed to ultraviolet light. They found that radical formation is controlled by cation effects on the overall structure and organization of the solution. Smaller cations bind to water more tightly, creating a closer packed solution. This makes forming radicals more difficult and leads to lower reactivity. The opposite was found for larger cations—the lower density solution network promotes radical formation and reactivity.
The Impact
Radicals are highly reactive chemical species that form under radiation. While the creation of radicals from nitrite solutions has been previously studied, prior work has focused on conditions very different from the extreme environment found in legacy tank wastes. By using highly concentrated and basic solutions, researchers found that the structure of the solution affects radical formation and the radical reactivity. This knowledge allows scientists to identify the origin of radical species and factors controlling their reactivity in concentrated, multicomponent solutions, helping to enable a predictive understanding of radiation effects.
Summary
Solutions of nitrite ions, when exposed to radiation, form a diverse range of radicals. These radicals participate in a wide range of chemical reactions, affecting the overall composition of a solution. Researchers studied how varying a metal cation altered the formation of nitrite-derived radicals in highly concentrated solutions. They found that the nature of the cation had a significant effect on the production of radicals under ultraviolet irradiation. Solutions with small, charge dense cations, such as lithium, had significantly diminished radical production. Solutions with larger and less charge dense cations, such as cesium, had increased production of radicals. The team found that the cations affect the overall structure of the solution due to their ability to bind water. The smaller cations tightly bind water, creating a tighter solution network that makes it more difficult for the radicals to move in the solution and slows down reactions. The traditionally proposed mechanisms and reactions leading to radical formation in nitrite solutions do not account for these important solution effects. Our results propose that solution structures and interactions must be accounted for when developing a predictive understanding of radiation effects in legacy tank waste.
Contact
Emily Nienhuis, Pacific Northwest National Laboratory, emily.nienhuis@pnnl.gov
Carolyn Pearce, Pacific Northwest National Laboratory, IDREAM EFRC Director, carolyn.pearce@pnnl.gov
Funding
This research was supported by IDREAM (Interfacial Dynamics in Radioactive Environments and Materials), an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (Grant No. FWP 68932). Electron paramagnetic resonance spectroscopy and Nuclear Magnetic Resonance spectroscopy were performed using facilities at EMSL, the Environmental Molecular Science Laboratory, a DOE Office of Science user facility sponsored by the Biological and Environmental Research program at Pacific Northwest National Laboratory.
Published: April 3, 2024
Nienhuis, E. T., Graham, T. R., D’Annunzio, N. L., Kowalska, M. I., LaVerne, J. A., Orlando, T. M., Reynolds, J. G., Camaioni, D. M., Rosso, K. M., Pearce, C. I., Walter, E. D. “Cations impact radical reaction dynamics in concentrated multicomponent aqueous solutions,” J. Chem. Phys. 158, 224503 (2023). DOI: 10.1063/5.0153132