Unlocking the Secrets of Aluminum Salt Crystallization
IDREAM’s novel insight to inform safer nuclear waste processing
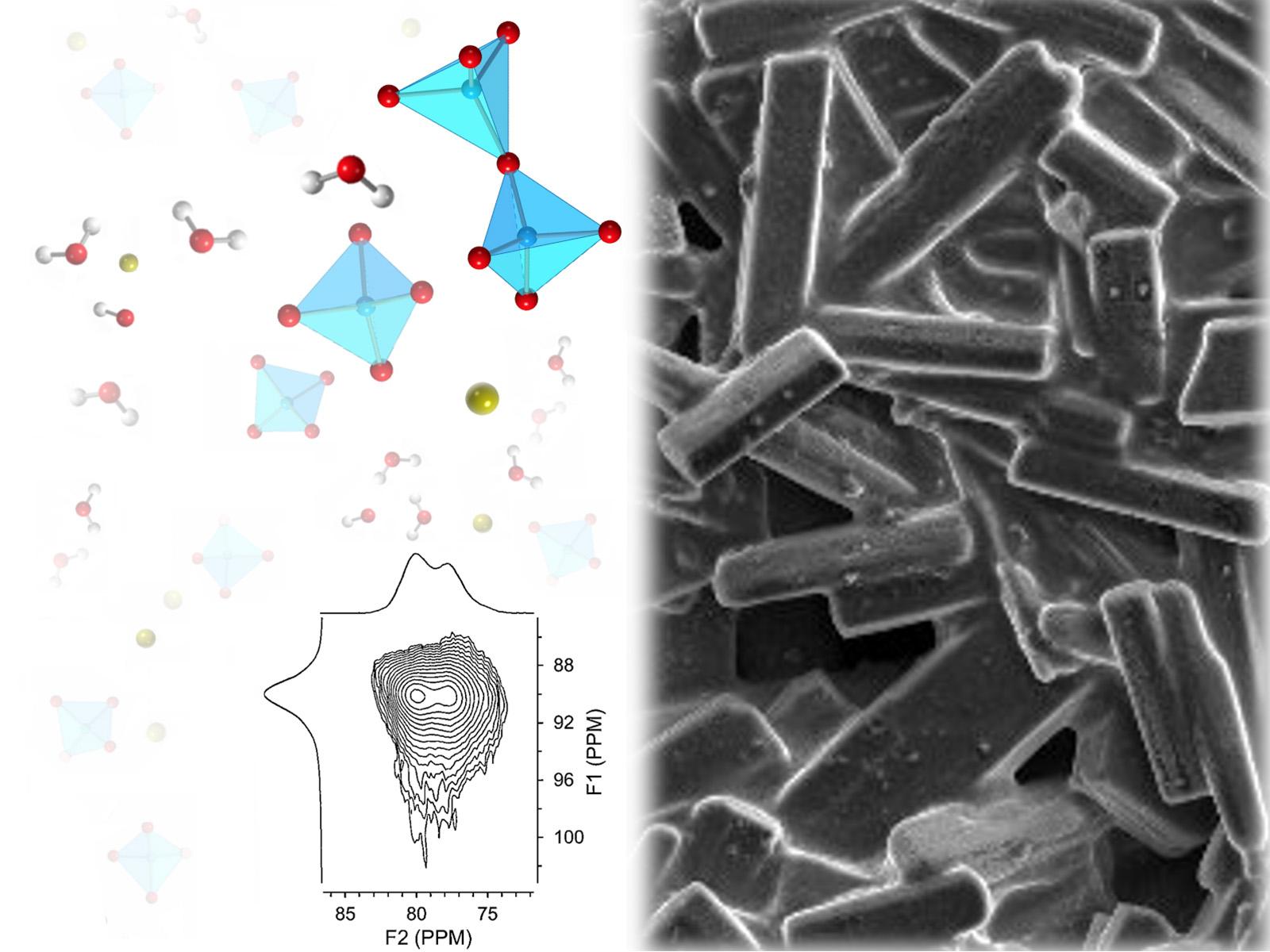
Intermediate Species in the Crystallization of Sodium Aluminate,” published in The Journal of Physical Chemistry C—is helping unlock the secrets of how aluminum salts and minerals are formed.
(Image: Adapted from J. Phys. Chem. C 2020, 124, 23, 12337-12345)
Legacy waste from nuclear weapons production has been stored for decades at the U.S. Department of Energy’s (DOE’s) Hanford Site in Washington State and Savannah River Site in South Carolina. The wastes have large quantities of aluminum salts and minerals, present in part due to the removal of aluminum from fuel cladding. The various phases of aluminum subsequently affect downstream nuclear waste processing, such as the formation of glass.
The Interfacial Dynamics in Radioactive Environments and Materials (IDREAM) Energy Frontier Research Center, a Pacific Northwest National Laboratory (PNNL)-led partnership, seeks to better understand the chemical reactions that occur when aluminum salts and minerals crystallize from solution. With these studies, IDREAM researchers are strengthening the technical understanding of aluminum solubility in radioactive tank waste. This knowledge can improve the efficiency of waste retrieval and processing operations while minimizing costs.
The Science
Dissolution, nucleation, and precipitation of aluminum phases are among the underlying chemical phenomena that are used to retrieve and process legacy waste from million-gallon tanks. The behavior of aluminum significantly affects these retrieval and processing operations, yet there is uncertainty in how it crystallizes and dissolves under different conditions. To make nuclear waste processing more efficient and reduce its overall cost, a study by IDREAM, published in The Journal of Physical Chemistry C, looked more closely at the crystallization and dissolution of aluminum phases.
Before this research, identifying aluminum oxyanion species that precede aluminum salt crystallization in highly alkaline “water-in-salt” solutions was challenging. The initial building blocks, or pre-nucleation species, such as monomeric and dimeric aluminate oxyanions, have similar chemical signatures, making it difficult to differentiate between them and determine the mechanisms of aluminum salt crystallization from solution.
To measure these pre-nucleation species, researchers performed nuclear magnetic resonance spectroscopy (NMR) on caustic aluminum salt hydrates at PNNL’s Environmental Molecular Sciences Laboratory.
The Impact
This first-of-its-kind NMR spectroscopy measurement demonstrates that water-in-salt solutions can retain lingering spectroscopic fingerprints of elusive pre-nucleation aluminum species, specifically dimeric aluminum oxyanions. The identification of these dimers in localized chemical environments, created during crystallization of aluminum salts when water is limited, suggest they may facilitate transition between aluminum present in caustic liquids and solid aluminum phases.
As DOE and others look to the continuing, safe retrieval and processing of legacy nuclear wastes, understanding the chemical speciation of aluminum in caustic environments will enable better selection of process conditions. This awareness allows control of both dissolution and crystallization of aluminum phases.
In addition to providing a more detailed understanding of aluminum chemical species in legacy nuclear wastes, this knowledge can be extended to the refining of metallic aluminum from mineral ores. Metallic aluminum obtained in this manner can be used in the production of a diverse range of consumer and industrial products.
Summary
In this IDREAM study, details of the mechanism controlling transformation of aluminum in solution to insoluble crystalline aluminum phases are revealed. The role of dimeric building blocks in the crystallization of aluminum salt hydrates from highly alkaline, concentrated electrolytes demonstrates that unique, localized chemical environments are created when water is limited. This causes perturbation of aluminate tetrahedral coordination in solution to facilitate transition to the octahedral species in the solid. These findings strengthen the technical foundation for how aluminum is processed at nuclear sites like Hanford and Savannah River.
Funding
This research was supported by IDREAM, an Energy Frontier Research Center funded by the DOE Office of Science, Basic Energy Sciences. NMR spectroscopy and supporting experiments were performed using the facilities at the Environmental Molecular Science Laboratory, a DOE Office of Science user facility sponsored by the Biological and Environmental Research program at PNNL.
Research Team
The IDREAM research team, based at Pacific Northwest National Laboratory, included Trent R. Graham, Mateusz Dembowski, Jian Zhi Hu, Nicolas R. Jaegers, Xin Zhang, Sue B. Clark, Carolyn Pearce, and Kevin Rosso.
The team’s findings, “Intermediate Species in the Crystallization of Sodium Aluminate,” published April 15, 2020, in The Journal of Physical Chemistry C, DOI: 10.1021/acs.jpcc.0c00205.
Published: September 1, 2021
Trent R. Graham, Mateusz Dembowski, Jian Zhi Hu, Nicholas R. Jaegers, Xin Zhang, Sue B. Clark, Carolyn I. Pearce, and Kevin M. Rosso. 2020. Intermediate species in the crystallization of sodium aluminate hydroxy hydrates. The Journal of Physical Chemistry C, 124 (23), 12337-12345 [DOI: 10.1021/acs.jpcc.0c00205].