Surface Defects and pH Alter Gibbsite Dissolution Mechanisms
Differences in water interactions lead to two gibbsite dissolution pathways modulated by solution acidity
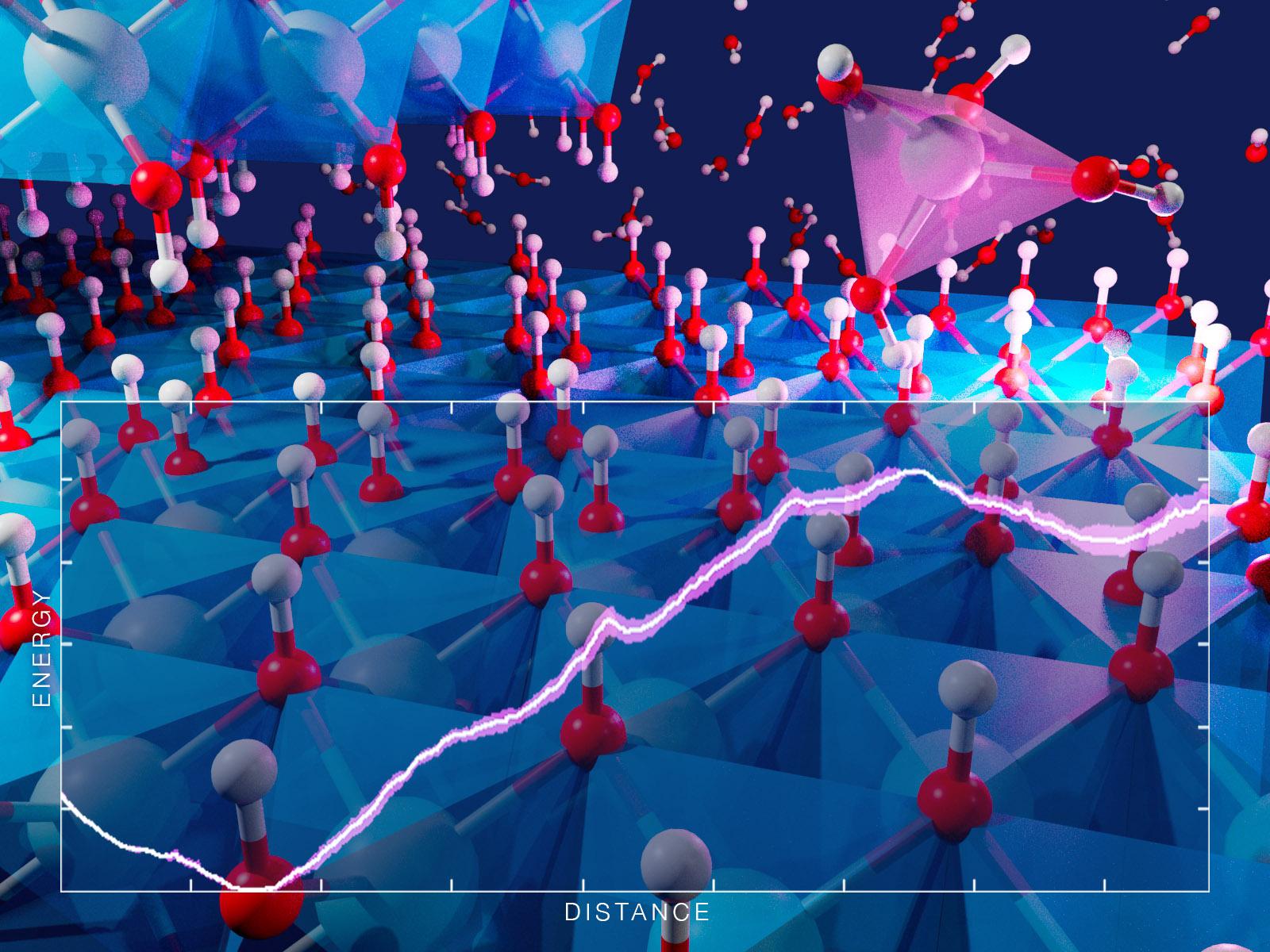
Aluminum can detach from the edges of gibbsite via two primary reaction pathways.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Mineral dissolution is a surface reaction facilitated by contact with water or other liquids. Natural weathering and industrial processing of minerals depend on dissolution. The initial chemical reactions often dictate how fast this process occurs. Researchers used ab initio molecular simulations to study how aluminum can detach from the edges of defect-laden gibbsite, an important mineral in the industrial processing of aluminum and radioactive tank waste. Two reactions that differ based on how much water is interacting with the detachment site were identified as important to dissolution. Changing the pH from neutral to alkaline conditions is predicted to greatly reduce the energy barrier, with the specific factors behind the change dependent on the edge morphology.
The Impact
The dissolution of gibbsite is central to some types of common industrial aluminum processing. Understanding how gibbsite dissolves can help researchers better process aluminum, including the aluminum-containing minerals found in legacy radioactive tank waste. By identifying different dissolution pathways, how those pathways depend on surface defects, and the underlying energetics of the reactions, scientists can better predict the solution conditions needed to control mineral dissolution and its speed.
Summary
Mineral dissolution commonly occurs in natural and industrial processes. However, the complex nature of the interface between a solid mineral and the surrounding liquid makes identifying molecular-scale mechanisms of dissolution a challenge. Researchers used ab initio molecular dynamics simulations to get a detailed picture of gibbsite dissolution pathways in both water and a sodium hydroxide solution. They explored aluminum monomer detachment from two types of gibbsite edges that represent different surface defects. The results showed that there are two potential reaction pathways for detachment in water that depend upon the amount of water bound to the aluminum-site. The mechanism and energy barriers for detachment change based on the pH of the solution.
PNNL Contact
Carolyn Pearce, Pacific Northwest National Laboratory, carolyn.pearce@pnnl.gov
Aurora Clark, University of Utah, aurora.clark@utah.edu
Funding
This research was supported by the Interfacial Dynamics in Radioactive Environments and Materials (IDREAM), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (FWP 68932). Molecular simulations used resources from the Center for Institutional Research Computing at Washington State University and the Center for High Performance Computing at the University of Utah.
Published: February 6, 2024
Guo, Q., M. Pouvreau, K. M. Rosso, and A. E. Clark. 2024. “Mechanisms of Dissolution from Gibbsite Step Edges Elucidated by Ab Initio Molecular Dynamics with Enhanced Sampling,” Geochimica et Cosmochimica Acta, 366, 201. [DOI: 10.1016/j.gca.2023.11.007]