Heavy Metal Separation through Ion Electrosorption at an Assembled Ionic Liquid-Water Interphase
A novel electrode functionalization strategy enables efficient removal of toxic heavy metals from wastewater
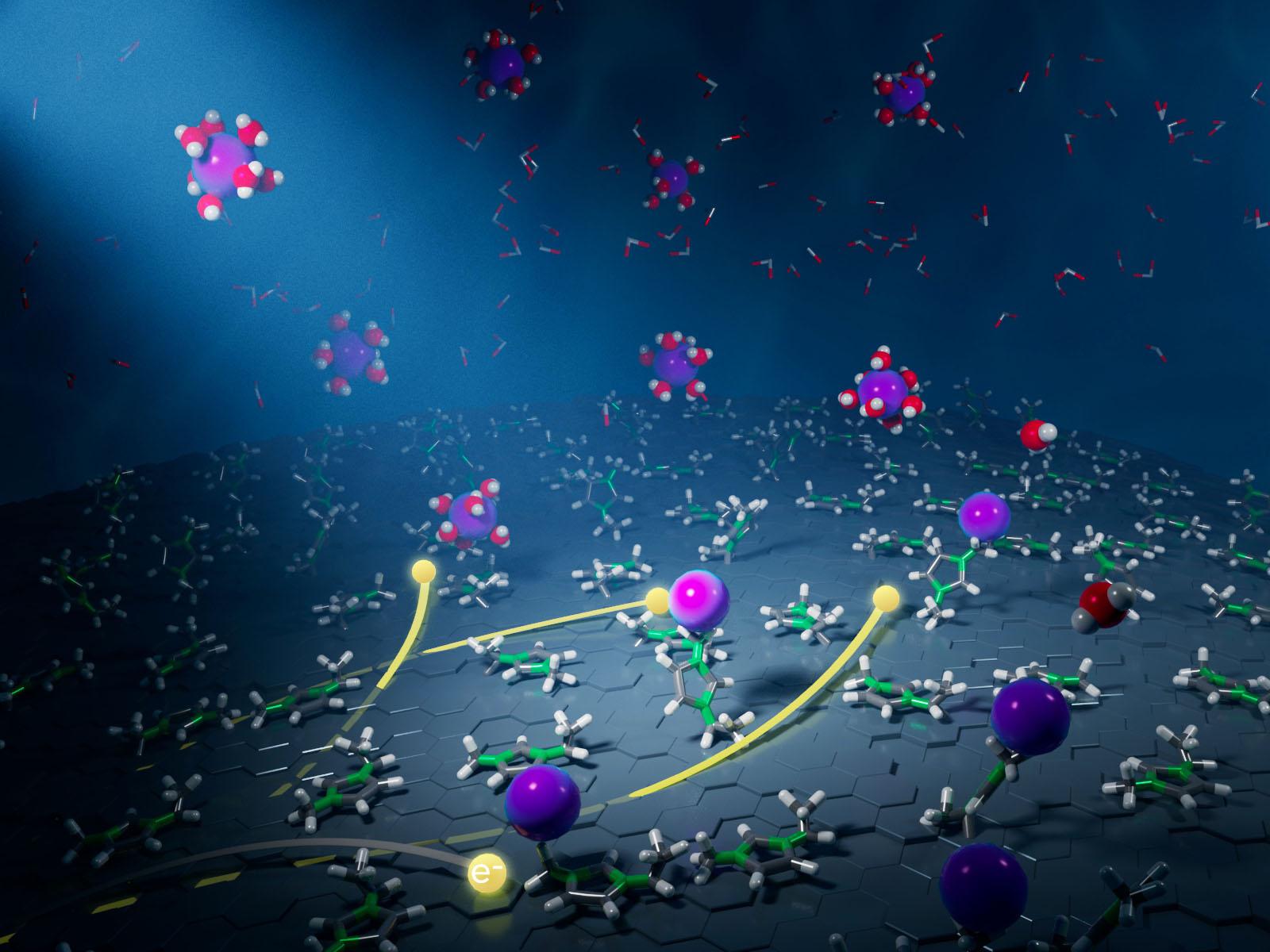
Adding small quantities of hydrophobic ionic liquids to waste streams increases the energy efficiency for removal of lead from bulk solution by a factor of two.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Traditional separation approaches often require high inputs of energy and are inefficient. An emerging paradigm of using electricity to modulate interfaces with tunable properties may enable highly efficient separations that use sustainably generated power. However, the complex chemistry at these interfaces remains challenging to understand and control. New research shows that adding a small amount of a hydrophobic ionic liquid to an aqueous system alters the properties of the electrode-electrolyte interface. A combined experimental and theoretical approach allowed researchers to identify well-oriented ionic liquid layers at the electrode that affect the nucleation of metal ions, leading to enhanced separation.
The Impact
Decontaminating industrial wastewater or recovering critical materials depends on having rationally designed, energy-efficient separation processes. Electrochemical separations are a promising approach to removing toxic heavy metals from waste streams, with relatively high selectivity and easy integration with sustainable energy sources. However, scientists don’t yet understand how to precisely control chemistry at electrified interfaces. This work shows that adding small quantities of hydrophobic ionic liquids to aqueous waste streams increases the energy efficiency for removal of lead from bulk solution by a factor of two. These results have substantial implications for designing next-generation separations processes.
Summary
Electrochemical separations based on electrified interfaces have exciting potential for the recovery of critical materials, the production of drinking water, and the remediation of polluted industrial sites. Despite the initial success of electrochemical separations, scientists do not understand how to control the chemistry between interfacial ions (e.g., electrolyte cations, anions) at interfaces well enough to achieve efficient desolvation and electroreduction of target ions. This research uses small quantities of hydrophobic ionic liquids in water to form electric-field-assembled interphases at an electrode-electrolyte interface. Through operando electrochemical Raman spectroscopy, atomic force microscopy, and electrochemical impedance spectroscopy combined with classical molecular dynamics simulations, researchers obtained kinetic and mechanistic insights into the metal ion desolvation and electrosorption properties of electric-field-assembled interphases. Applying a negative potential results in the formation of a well-oriented ionic liquid layer on the electrodes with the hydrophobic alkyl chains of the imidazolium cations extending into the solution. This arrangement facilitates desolvation of lead cations, decreases the charge transfer resistance for reduction, and controls the morphology of the electrodeposits that form. The results have substantial implications for the rational design of functionalized electrode-electrolyte interfaces that enable efficient water purification and selective recovery of critical materials.
PNNL Contact
Venky Prabhakaran, Pacific Northwest National Laboratory, venky@pnnl.gov
Grant Johnson, Pacific Northwest National Laboratory, grant.johnson@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division’s Separation Science program, project 72353 (Interfacial Structure and Dynamics in Separations). Part of this work was performed using EMSL, the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE Office of Science’s Biological and Environmental Research program and located at Pacific Northwest National Laboratory (PNNL). Computer resources were provided by Research Computing at PNNL and the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science user facility operated under contract DE-AC02-05CH11231.
Published: November 14, 2023
Tan S, Nguyen M-T, Zhang D, Zhong L, Cheng Z, China S, Johnson G E, Prabhakaran V, “Electric-Field-Induced Assembly of an Ionic Liquid–Water Interphase Enables Efficient Heavy Metal Electrosorption,” ACS Appl. Mater. Interfaces, 15, 44469–44481 (2023). [DOI: 10.1021/acsami.3c07465]