Visualizing Oxygen Transport Pathways Along Oxide/Metal Interfaces
Oxide/metal interfaces act as the primary path for oxygen to move to and around nanoscale protective oxide layers
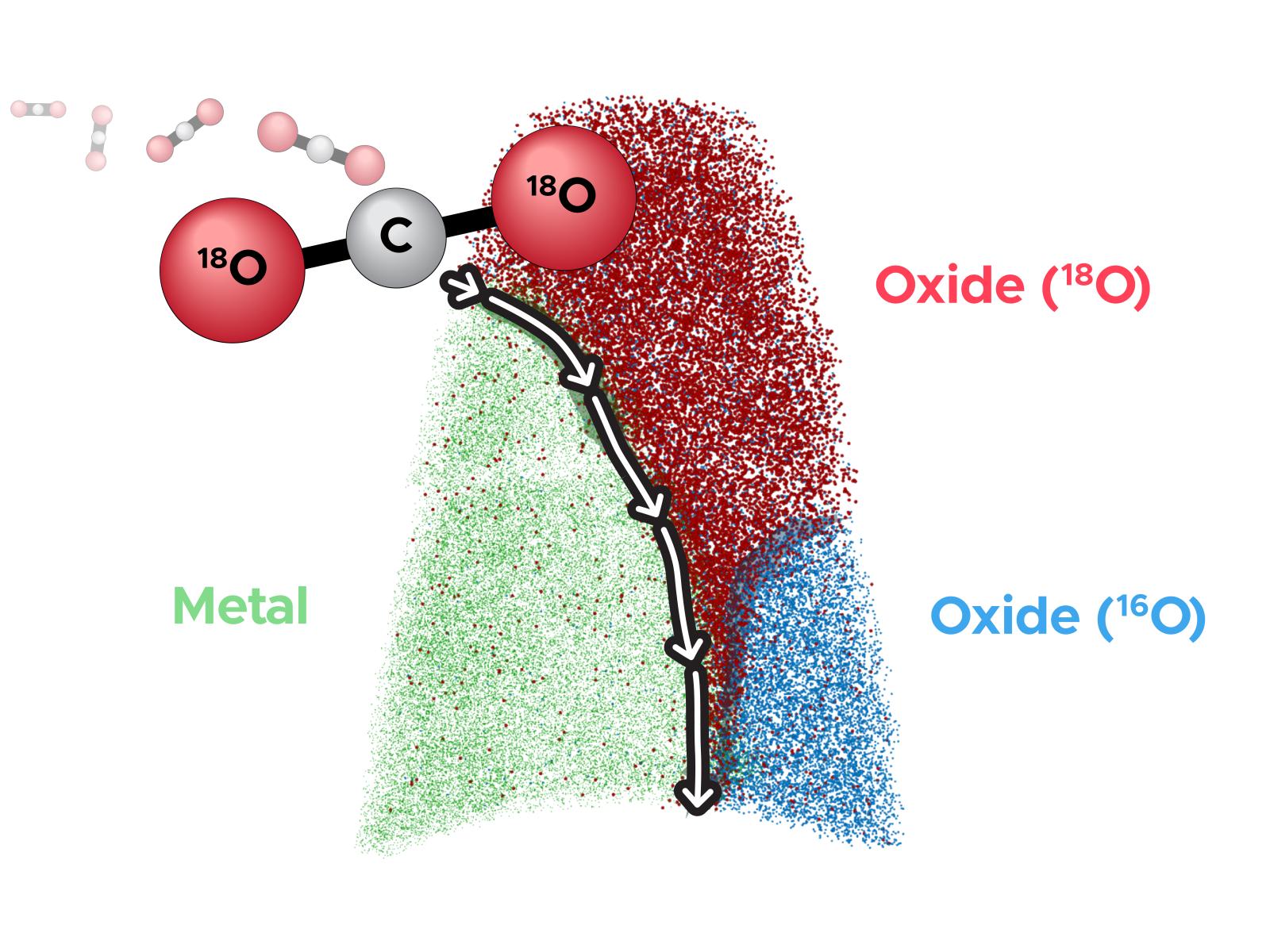
Atom probe tomography reveals that initial oxides (blue 16O-rich oxide) block most ingress of new oxides (red 18O-rich oxide). However small amounts of 18O trickle along the 16O-oxide/metal interface, enabling continued oxide growth.
(Image by Stephanie King | Pacific Northwest National Laboratory)
The Science
Alloys used in energy production are often exposed to hostile environmental conditions that cause corrosion and oxidation, degrading the alloy’s structural stability. To function safely, these materials must resist corrosion which is often achieved by slowing the atomic-scale transport of atoms between the environment and bare alloy. In corrosion resistant alloys, nanoscale oxide layers act as an effective barrier to oxidation and atom movement but still have defects that can enable ongoing corrosion. In this study, researchers used a two-stage oxidation process with different oxygen isotopes (16O and then 18O) and advanced imaging to identify the specific defects that enable this atomic movement, proving that oxide/metal interfaces are the main defect.
The Impact
Highly stable corrosion and oxidation resistant alloys are necessary to create effective and long-lasting energy generation systems. This work identifies a major mechanism of alloys oxidation that contributes to their degradation, highlighting the key weakness of current materials. With this additional information, researchers can begin identifying mitigation strategies during synthesis and material design.
Summary
Corrosion and oxidation resistant alloys are critical to maintaining the structural stability of components in high-temperature, aggressive environments that are common to energy generating systems. Nanoscale oxide layers that effectively block further movement of oxygen atoms into the metal or metal atoms toward the environment make this corrosion resistance possible. However, all passivating oxide layers contain structural flaws that can facilitate oxygen and metal transport to continue corrosion. Researchers used two different isotopes of oxygen (16O and 18O) in combination with advanced microscopy to understand how oxygen penetrates an otherwise highly protective oxide layer in model Ni-Cr alloys. The model alloys were first oxidized in a controlled CO/CO2 environment at 600°C using 16O-rich CO2, forming a protective oxide layer with ~99.8% 16O. The same material was then further oxidized in the same CO/CO2 environment, but highly 18O enriched (~98% 18O). Using the isotopic sensitivity of atom probe tomography (APT), 3D imaging delineated oxides formed in the 16O- and 18O-rich conditions. Analyses proved that very little 18O diffused into the 16O-rich oxides; rather the 18O enriched and diffused along oxide/metal interfaces to form new 18O-rich oxides. Although this mechanism has long been postulated, these observations are the first to definitively reveal the 3D nature and role of these structural defects in ongoing oxidation through protective oxide layers. This opens the door to new alloy designs that could potentially seek to block these fast diffusion pathways in the future.
PNNL Contact
Daniel Schreiber, Pacific Northwest National Laboratory, Daniel.Schreiber@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE) Office of Science, Basic Energy Sciences Materials Science and Engineering Division, Mechanical Behavior and Radiation Effects program (FWP 56909). MW acknowledges support from the Deutsche Forschungsgemeinschaft for a one-year research fellowship at Pacific Northwest National Laboratory, and support from the EAM, an FAU Competence Center, through Starting Grant EAM-SG21-3 for supplemental data analysis and manuscript preparation. Access to APT instrumentation was provided via a user proposal (10.46936/cpcy.proj.2019.51178/60006716) from EMSL, the Environmental and Molecular Sciences Laboratory, a DOE Office of Science user facility at Pacific Northwest National Laboratory.
Published: September 27, 2023
Weiser W., M. J. Olszta, M. H. Engelhard, Z. Zhu, and D. K. Schreiber. 2023. “Visualizing oxygen transport pathways during intergranular oxidation in Ni-Cr.” npj Materials Degradation 7, 70. [DOI: 10.1038/s41529-023-00387-w]