Selective Targeting of Triglycerides and Ceramides Prevents MERS-CoV Replication
Researchers use multiomics to confirm role of lipid synthesis during infection, leading to model of how to restrict virus replication
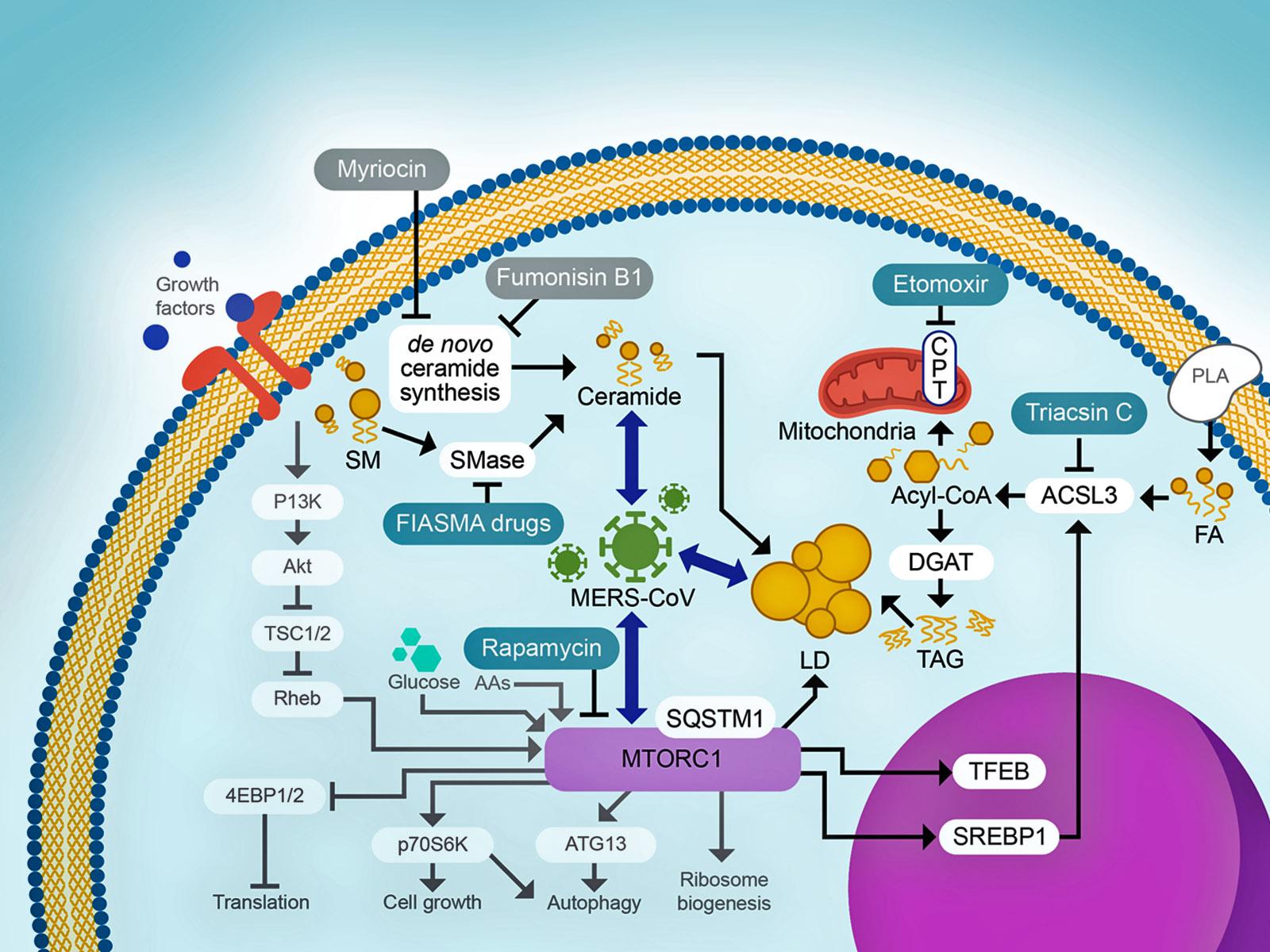
Model of lipid related cell signaling during MERS-CoV infection.
(Illustration by Hugh Mitchell and Stephanie King | Pacific Northwest National Laboratory)
The Science
Host lipid structures and abundances are observed to be significantly altered during infection, but the mechanisms regulating lipid synthesis and modification during coronavirus infection remain largely unknown. In this study, Pacific Northwest National Laboratory (PNNL) researchers analyzed a large multiomic dataset from three Middle East respiratory syndrome coronavirus (MERS-CoV)-infected primary human lung cell types—derived from three distinct donors—to investigate the role of lipid alteration during infection. Researchers confirmed that synthesis of triglycerides and ceramides are key to virus replication, suggesting that by inhibiting specific subsets of lipid metabolism, the virus can be stopped. The research is part of an internal investment at PNNL called the Predictive Phenomics Initiative, which is focused on understanding the function of complex biological systems so they can be engineered for other uses.
The Impact
Combating emerging viral threats requires an in-depth understanding of how the virus commandeers host resources to facilitate replication. Viral particles are comprised of proteins and lipids so the synthesis of each component is critical for viral replication. PNNL researchers have demonstrated the importance of the synthesis of two lipid species, ceramides and triacylglycerides, for MERS-CoV replication and that virus replication is not successful if these synthesis pathways are blocked.
Summary
Researchers investigated the role of lipid synthesis pathways during MERS-CoV infection of primary human lung cell types derived from three distinct donors. From observations of perturbations of various lipid classes using lipidomics data, the researchers hypothesized and confirmed that infection orchestrates an increase in ceramide species via sphingomyelinase pathways required for infection. They also identified a minor subset of lipid-related proteins with increased differential expression among a striking majority of lipid-related proteins with decreased differential expression. Of the proteins identified the most prominent was ACSL3, a long-chain acyl-CoA synthetase, that is key for synthesis of triacylglycerol, which is also known to be associated with lipid droplet formation, an established feature of coronavirus-infected cells. Researchers demonstrated that inhibition of acyl-CoA synthetase activity blocked MERS-CoV replication.
In addition, perturbation of lipid dependent functions in transcriptomics and proteomics suggested alterations in mTORC (mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1)-related pathways and future studies will further explore mTORC’s role in MERS-CoV replication . These results suggest a model wherein coronaviruses perturb overall cellular metabolism to shift resources to the production of ceramides and triglycerides, in particular through acyl-CoA synthetase activity. The findings suggest a strategy for targeting CoV replication through inhibition of specific subsets of lipid metabolism.
Contact
- Amy Sims, amy.sims@pnnl.gov, PNNL
- Hugh Mitchell, hugh.mitchell@pnnl.gov, PNNL
Funding
The research described in this paper is part of the Predictive Phenomics Initiative at PNNL and conducted under the Laboratory Directed Research and Development Program. PNNL is a multiprogram national laboratory operated by Battelle for the Department of Energy under Contract No. DE-AC05-76RL01830.
Published: January 16, 2026
Mitchell HD, J Kyle, K Engbrecht, M Berger, KL Oxford, AC Sims. 2026. Increased triacylglyceride and ceramide levels are key for MERS-CoV replication. mSphere 0:e00523-25. https://doi.org/10.1128/msphere.00523-25