Resolving Proton Dynamics in Water
Combining transition state theory with Marcus theory provides insight into the fundamental processes of proton transfer in water
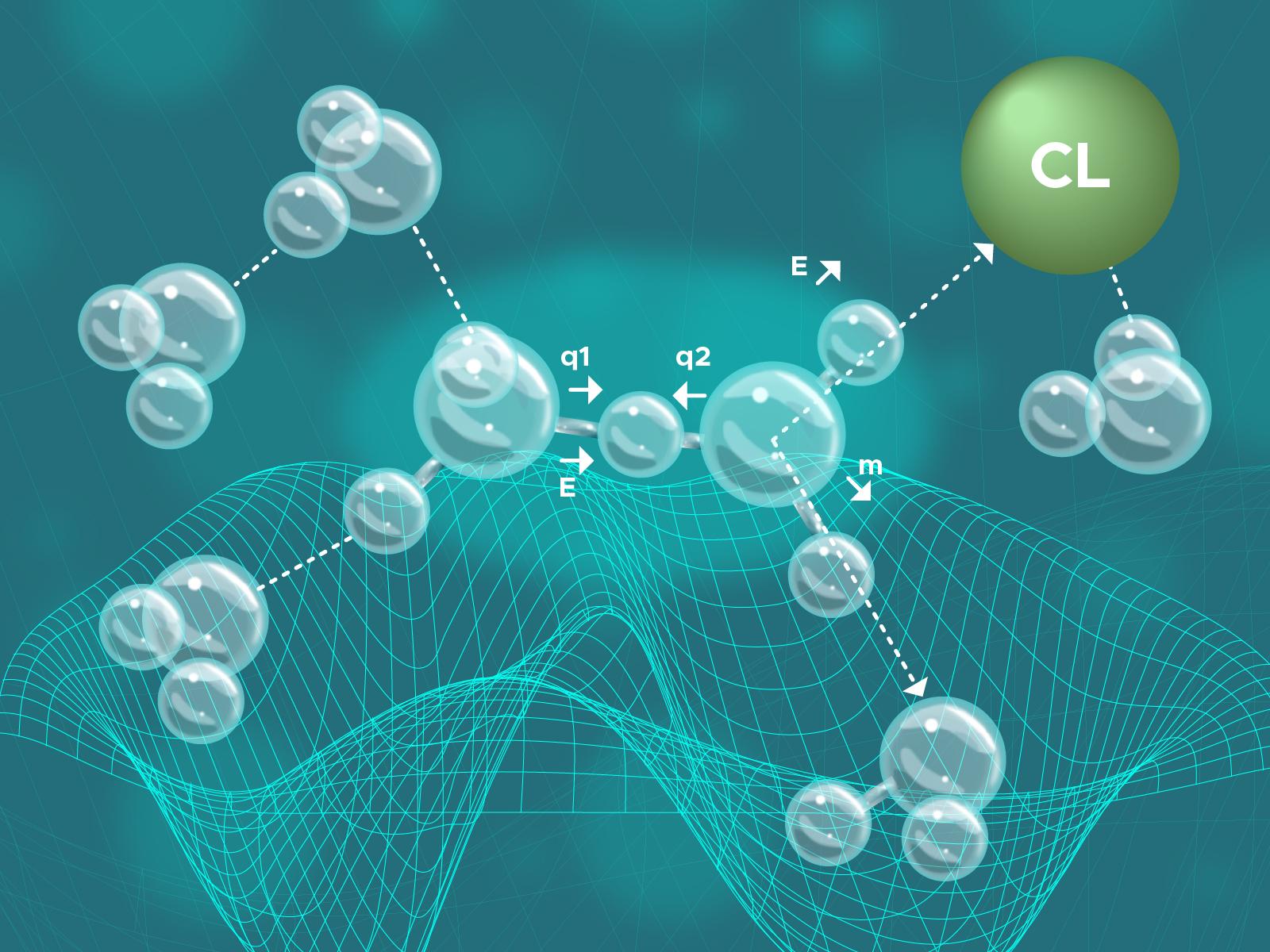
Schematics of the 2D reaction coordinate: a proton shuttles between two water molecules in the Zundel state as it dissociates from Cl- in an aqueous HCl solution.
(Illustration by Stephanie King | Pacific Northwest National Laboratory)
The Science
Despite hydrogen being the smallest and most abundant element in the universe, scientists have yet to fully understand its behavior. When in water, hydrogen ions interact with surrounding water molecules through transient bonds that are continually broken and re-formed. This collective motion adds complexity to the behavior of these particles.
In this study, researchers combined Marcus theory, which describes the rate of electron transfer, and transition state theory, which explains the rate of chemical reactions, to understand the motions, fluctuations, transport, and rearrangement of positive hydrogen ions, or protons, in water.
The Impact
The motions of hydrogen ions control a variety of processes and phenomena. The equilibria, fluctuations, and reactivity of excess protons in water are responsible for acid/base chemistry that affects our lives in many ways. As the lightest chemical element, hydrogen provides challenges in our ability to predict its behavior. This research provides insight into the fundamental processes of proton transfer in water by combining transition state theory with Marcus theory. These findings can be applied to various systems including energy storage in batteries and chemical processing of materials.
Summary
Marcus theory was originally developed by Nobel Laureate Rudolph A. Marcus to describe the rate of electron transfer in chemistry. Transition state theory is a powerful framework for predicting rare events. To understand proton dynamics in water, researchers combined these theories into a single framework applied to understand the separation of H+ and Cl- in a concentrated HCl solution, then modeled this motion using ab initio molecular dynamics simulations. The scientists also considered the complexities associated with the collective motions of water.
The researchers applied unconventional descriptors to track and predict the motion of these protons, such as the vibrational frequency of bonds as well as the instantaneous electric fields at atomic sites. In doing so, they proposed an explanation for the concentration dependence of the rate of proton transfer that has been verified by experimental studies. This discovery uncovered the significance of collective motion limiting the transfer of energy during the proton transfer process. These findings also provide a better understanding of the role of spectator ions and electrolyte concentration effects. Future work will extend these findings to more complex systems.
Contact
Gregory Schenter
Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory
greg.schenter@pnnl.gov
Funding
- Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences.
- Materials Synthesis and Simulation Across Scales (MS3) Initiative, a Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory (PNNL).
- National Science Foundation.
- Camille Dreyfus Teacher-Scholar Awards Program.
Published: April 13, 2021
S. Roy, et al., 2020. “Resolving Heterogeneous Dynamics of Excess Protons in Aqueous Solution with Rate Theory.” Journal of Physical Chemistry B, 124, 27:5665-5675. [DOI: 10.1021/acs.jpcb.0c02649]