Organic Molecule Mimics Behavior of Alkali Metals in Electron Transfer Reactions
A common organic host molecule behaves like alkali metals, enabling long-range electron transfer harpoon reactions with halogens
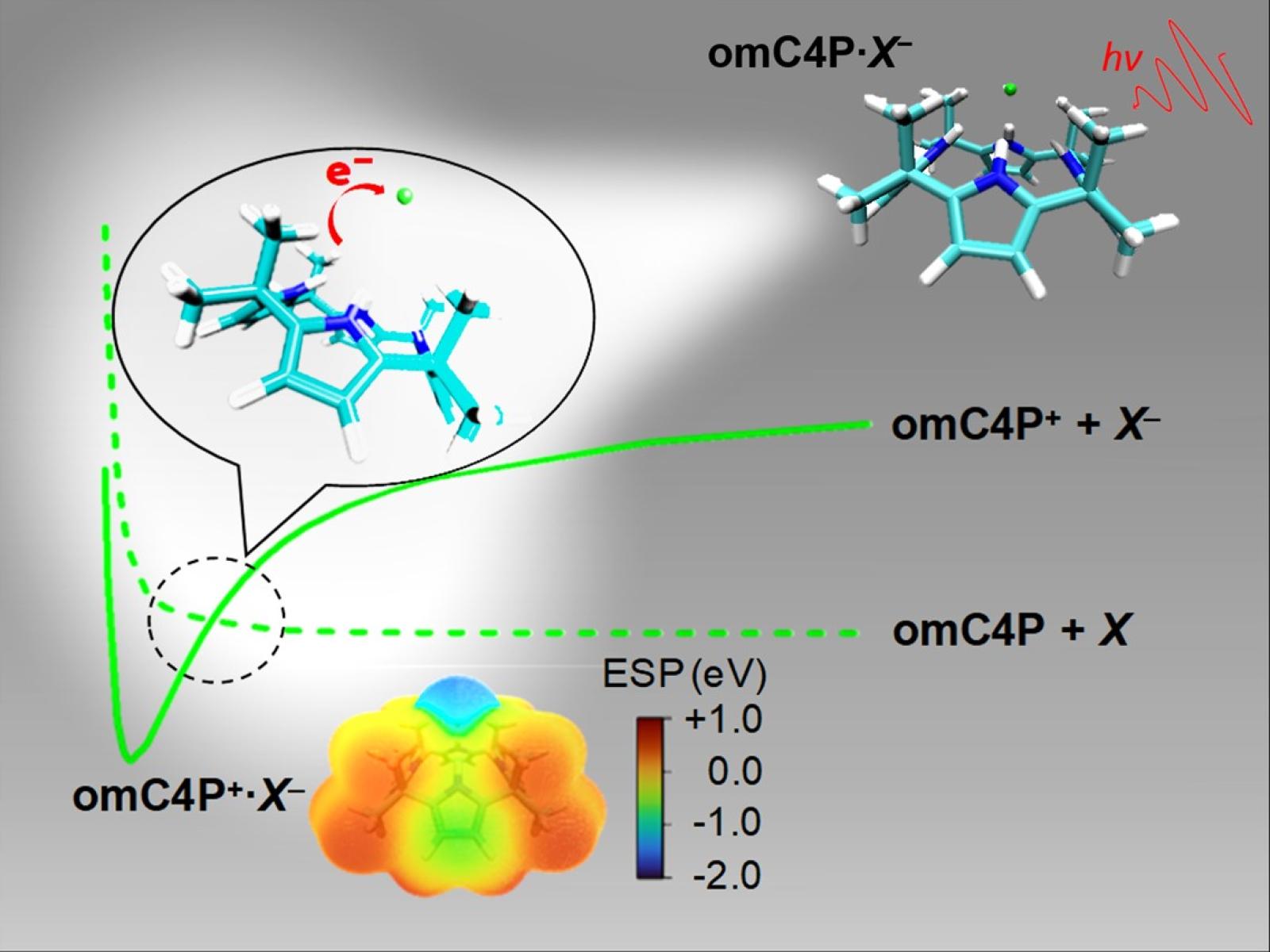
New research shows that an organic host molecule can mimic the behavior of alkali metals, spontaneously enabling long-range electron transfer processes.
(Image by Wenjin Cao | Pacific Northwest National Laboratory)
The Science
Long-range electron transfer reactions play important roles in various chemical and biochemical processes. Until now, common understanding was that only alkali metals possessed the capability to spontaneously enable long-range electron transfer harpoon reactions with halogens. This study demonstrates that a popular and versatile organic host molecule, octamethylcalix[4]pyrrole (omC4P), can behave like an alkali metal and react with various halogens and pseudohalogens (X) to form charge-separated omC4P+·X– complexes.
The Impact
This work reveals, for the first time, that common organic molecules can mimic alkali metals in long-range electron transfer harpoon reactions. Given the broad existence of organic molecules, the finding that they can promote long-range electron transfer processes could provide crucial yet missing links to better understand the complexity of biological electron transfer reactions and ionic chemical bond formations in synthetic material sciences.
Summary
While long-range electron transfer harpoon reactions play a vital role in complex biological electron transfer reactions and ionic chemical bond formation, until now, only alkali metals were thought to be capable of them. For the first time, this work demonstrates that the organic molecule omC4P can mimic the behavior of alkali metals, achieving long-range harpoon electron transfer reactions with halogens and pseudohalogens. This work combines high-precision cryogenic photoelectron spectroscopy with an electrospray ionization source, a one-of-a-kind instrument developed at Pacific Northwest National Laboratory, and high-level quantum chemical computations. The electronic structure and chemical bonding information of the neutral complexes was characterized by recording photoelectron spectra of the corresponding omC4P·X– anions, which revealed that the ground state of the neutrals is in the charge-separated omC4P+·X– configuration.
The widespread omC4P organic host molecule reacts with various halogens or pseudohalogens to form charge-separated omC4P+·X– complexes. This occurs via electron harpooning from omC4P to X at a distance that correlates with the energy difference between the ionization potential of omC4P and the electron affinity of X. This work opens doors to further research into non-metal harpoon reactions, which could be tested on additional organic candidates. In addition, the dynamics of harpoon reactions between organic molecules and halogens could be further investigated.
Contact
Xue-Bin Wang, Pacific Northwest National Laboratory, Xuebin.Wang@pnnl.gov
Funding
This work was funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program; Division of Chemical Sciences, Geosciences, and Biosciences; Condensed Phase and Interfacial Molecular Science program, FWP 16248.
Published: May 28, 2024
Cao W, Wang XB. Organic molecules mimic alkali metals enabling spontaneous harpoon reactions with halogens. Chem. Eur. J. 2024; 30: e202400038. [DOI: 10.1002/chem.202400038]