Ionic Liquids Tune Graphene Oxide Membranes for Efficient Separations
Binding ionic liquids to thin layers of graphene oxide produces more selective and efficient multi-layer membranes for water filtration
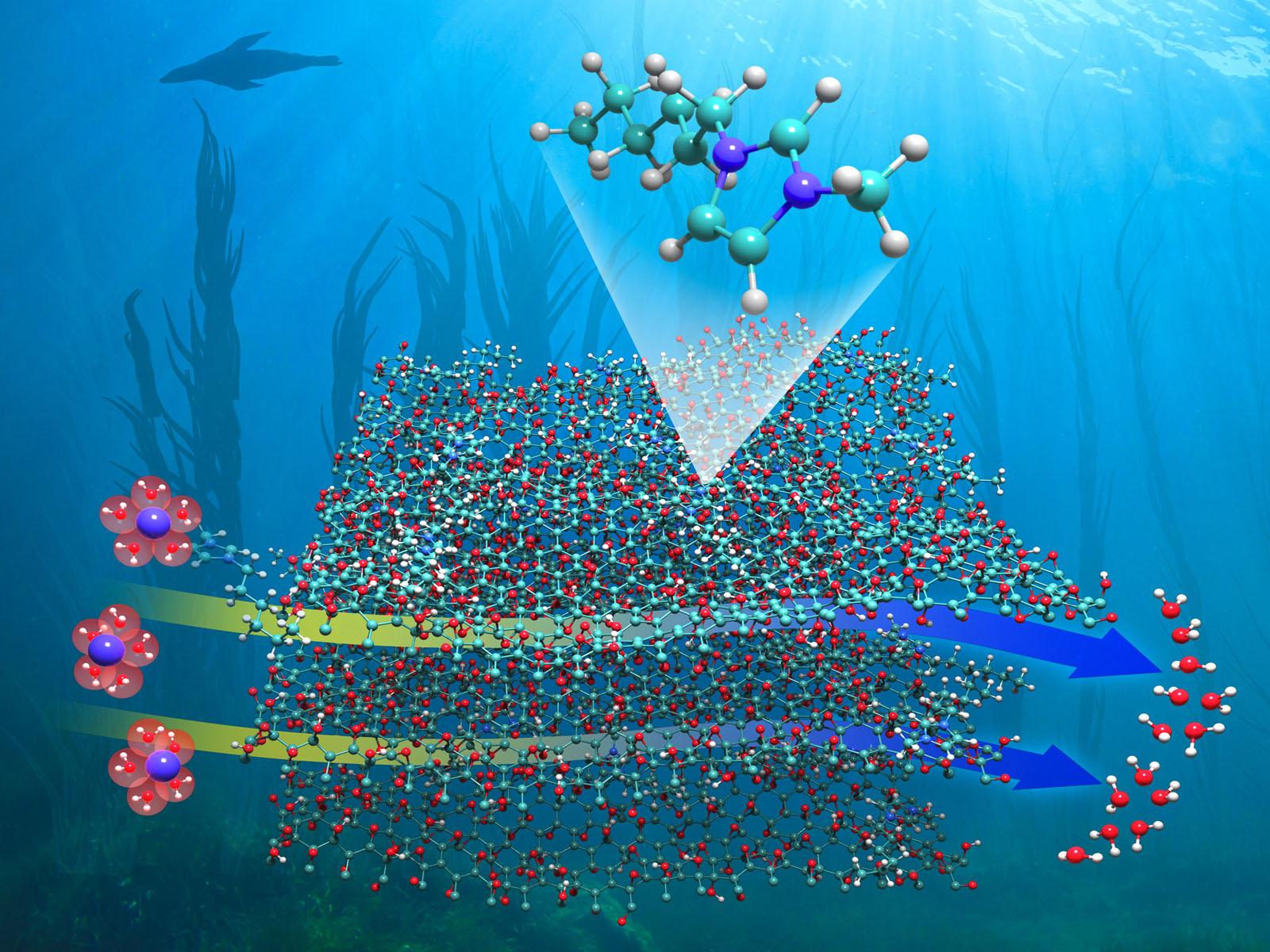
Functionalizing graphene oxide with imidazolium-based ionic liquids enables the preparation of separation membranes that efficiently filter out ions from solution with increased water flow.
(Image by Mike Perkins | Pacific Northwest National Laboratory)
The Science
Graphene oxide (GO) is a promising material for constructing separation membranes because it is straightforward to fabricate, mechanically strong, and has a low-cost to scale up production. However, the relatively low water flow rate through current GO membranes limits their use in the production of purified water, the recovery of critical elements, and environmental remediation. Researchers used the unique properties of imidazolium-based ionic liquids (ILs) to chemically modify GO membranes at their surface epoxide sites. By tuning the hydrophobicity and surface charge of the GO membranes, both the selectivity of ion removal and water flow rate were enhanced in the IL-functionalized membranes.
The Impact
This new approach to tuning the selectivity and efficiency of GO membranes involves bonding ILs to the surface epoxide functional groups of GO under mild experimental conditions. This work demonstrates that the presence of imidazolium-based ILs enhances the ability of GO membranes to separate ions from solution. Changing the length of the carbon chain on the ILs tailors the flow of water through the membrane without compromising its capacity for ion adsorption and rejection, providing a precise method of tuning membrane properties. These novel IL/GO membranes show promise for efficiently separating ions and recovering critical materials used in advanced energy technologies.
Summary
GO membranes show promise for separating ions and recovering critical materials. However, the use of GO membranes is currently limited by low flow rates as water molecules diffuse through the membrane layers and associated hydrogen bonding network. Traditional methods of GO functionalization are through the surface carboxylic acid groups, which limit the dispersion of GO in water necessary for fabricating multilayer membranes. Researchers immobilized imidazolium-based ILs on GO via an epoxide ring-opening reaction that results in the formation of a hydrogen bond. As a result, the IL modified GO membranes disperse well in water, enabling uniform and reproducible preparation of high-performance multilayer membranes. The joint experimental characterization and theoretical calculations revealed that the geometric and electronic properties of the imidazolium cations simultaneously alter the hydrophobicity and surface charge of the GO membranes. The modification leads to increased efficiency separating different metal and chloride ions from a solution compared to bare GO. The positively charged imidazolium groups of the IL repel positively charged metal cations, resulting in a sieving process that efficiently separates ions based on charge. Additionally, increasing the length of the carbon chain of the IL increased the hydrophobicity of the membrane. This increase then enabled fine-tuning of the water desolvation and transport dynamics at the GO/IL interface. During ion separation the water flow through the membranes was high. These tunable parameters highlight the potential of IL functionalized GO membranes for a range of important separations, including water treatment, recovery of critical minerals, and environmental cleanup.
PNNL Contact
Grant Johnson, Pacific Northwest National Laboratory, grant.johnson@pnnl.gov
Venky Prabhakaran, Pacific Northwest National Laboratory, venky@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences program, Chemical Sciences, Geosciences, and Biosciences Division, project 72353 (Interfacial Structure and Dynamics in Separations). Part of this work was performed at EMSL, the Environmental Science Laboratory, a DOE Office of Science user facility at Pacific Northwest National Laboratory. Computer resources were provided by Research Computing at Pacific Northwest National Laboratory and the National Energy Research Scientific Computing Center.
Published: April 27, 2022
Tan S., D. Zhang, M-T. Nguyen, V. Shutthanandan, T. Varga, R. Rousseau, G.E. Johnson, V-A. Glezakou, and V. Prabhakaran. “Tuning the Charge and Hydrophobicity of Graphene Oxide Membranes by Functionalization with Ionic Liquids at Epoxide Sites,” ACS Applied Materials and Interfaces, (2022). [DOI: 10.1021/ascami.2c02366]