Environment Does Not Determine Light-Driven Organic-Nanoparticle Electron Exchange Efficiency
The nature of an iron oxide mineral and amount of light control electron movement from a light-absorbing molecule to the mineral, rather than the conditions in the surrounding aqueous environment
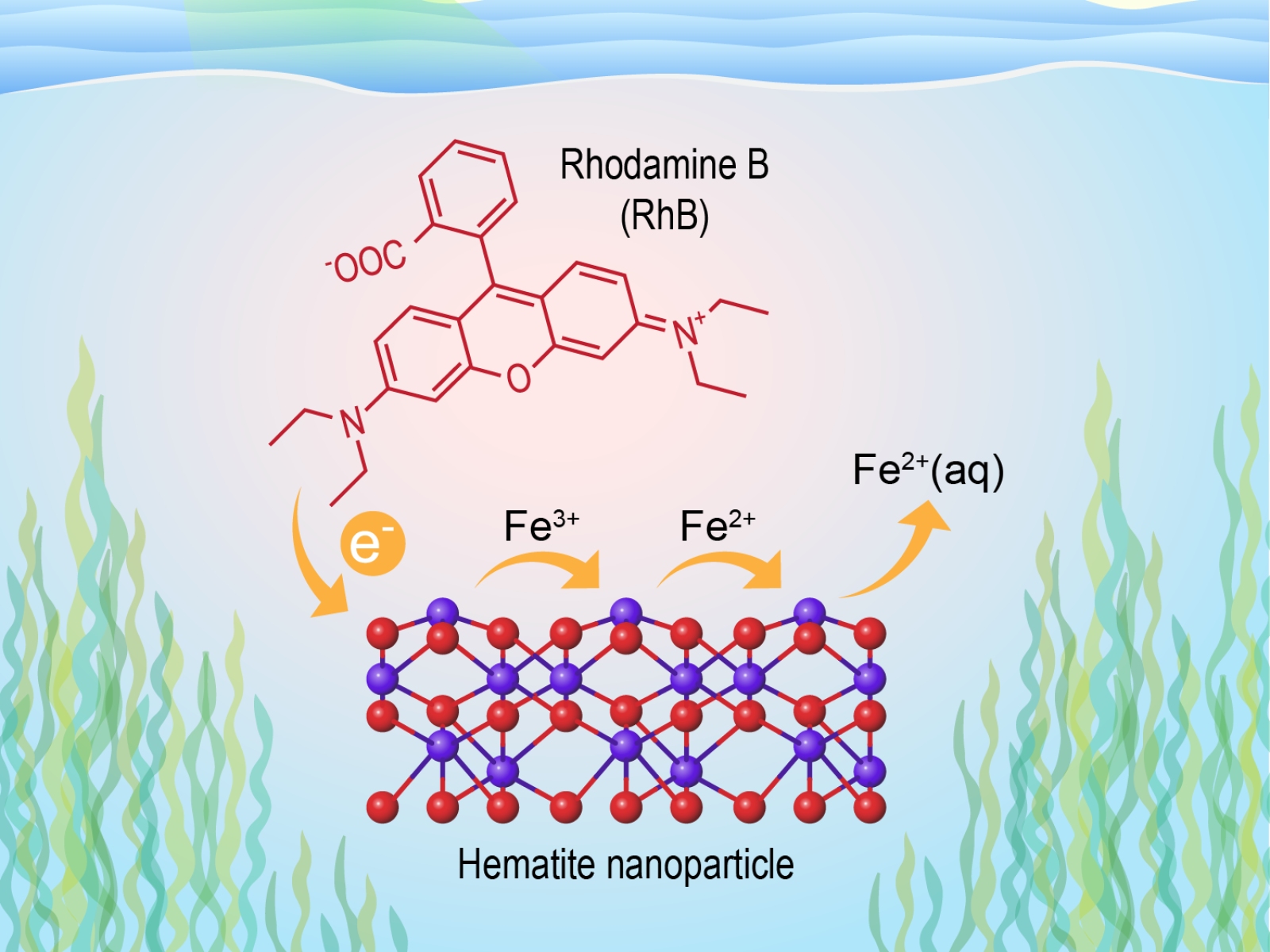
Light-induced reductive dissolution rates of iron oxide nanoparticles coated with organics, common in the sunlit zone of aquatic systems, mainly depend on the physicochemical nature of the mineral particles rather than the surrounding solution conditions.
(Illustration by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Electron transfer reactions that facilitate the breakdown of solid minerals are essential for nutrient availability and contaminant transport in natural systems. Such reactions tend to be sensitive to solution conditions such as pH. However, these reactions are challenging to investigate because they happen on ultrafast timescales. Researchers used nanoparticles of the iron oxide known as hematite (HNPs) and a common light-absorbing dye molecule (rhodamine B, RhB) to produce a light-induced charge transfer that mimics organic-coated nanoparticles in nature. They then employed time-resolved spectroscopy to quantify the efficiency of charge transfer when excited by light. They also explored the influence of pH on the reaction, concluding that the rates of electron transfer mainly depend on the nature of the mineral particles and available light rather than the solution environment.
The Impact
Understanding what role the environment plays in transforming and releasing nutrients and contaminants from nanoparticles is essential for developing practical solutions to clean energy and water sustainability challenges. This work demonstrates that the aqueous environment surrounding HNPs plays a surprisingly limited role in the electron transfer reactions that lead to particle dissolution. The findings have implications for the top layers of natural aquatic systems where similar iron oxide nanoparticles coated with natural organic matter exist. The results presented here can help shape the direction of basic science research related to nanomineral-based devices that will be introduced into the environment.
Summary
The electron transfer processes that underlie the light-driven reductive dissolution at interfaces between nanoparticles, organic molecules, and the solution occur on difficult to probe ultrafast timescales. Time-resolved spectroscopic studies have steadily evolved to enable investigations of interfacial photocatalyzed electron transfer efficiency from bound organic light absorbers to iron oxide particles. This study builds on current knowledge by examining a broader pH range, exploring the effects of solvent type and particle loading, and focusing on structurally well-defined HNPs. Comparing the measured transient absorption behavior of pure RhB solutions, HNP suspensions, and their binary mixtures as a function of particle loading and pH reveals clear evidence for electron injection into HNPs. Under select conditions, researchers found that RhB photoexcitation is longer-lived when bound to HNP surfaces, consistent with electron injection into the HNPs that delays electron transfer back to RhB and the corresponding relaxation of the dye. The findings help shed new light on factors controlling the interplay between photoexcitation of light-absorbing organics on metal oxide particles, their semiconducting properties, and the influence of critical environmental variables such as pH. The results are also relevant to conceptually similar research objectives in developing dye-sensitized photovoltaic devices for light-to-electrical energy conversion.
PNNL Contact
Mavis Boamah, Pacific Northwest National Laboratory, mavis.boamah@pnnl.gov
Kevin Rosso, Pacific Northwest National Laboratory, kevin.rosso@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences program, Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences Program at Pacific Northwest National Laboratory (PNNL). Mavis Boamah acknowledges support from the Linus Pauling Postdoctoral Fellowship program at PNNL. A portion of the work was performed using EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at PNNL.
Published: December 2, 2022
M. D. Boamah, X. Huang, A. Joly, Z. Wang, and K. M. Rosso. 2022. “Photocatalyzed Electron Exchange between Organic Chromophores and Hematite Nanoparticles and the Role of Solid-State Charge Transport,” Environ. Sci.: Nano, 9, 4119-4135. [DOI: 10.1039/d2en00567k]