Developing New Computational Methods for Atomic-to-Mesoscale Chemistry
A hybrid model effectively reproduces key observations of chemical systems while maintaining computational efficiency
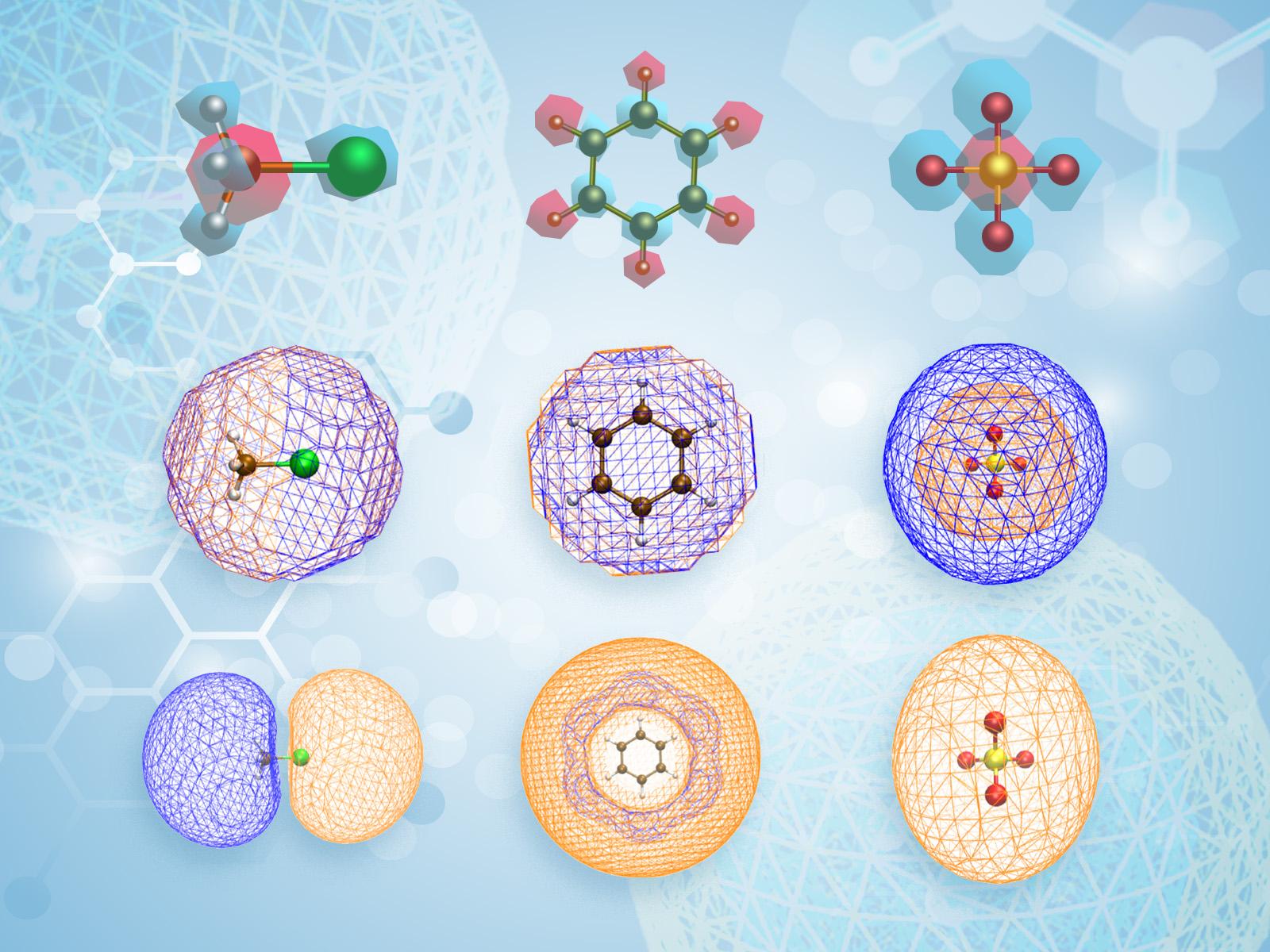
A new study demonstrates a hybrid model that can simulate part of a system at the molecular scale and other parts at larger scales in a computationally efficient manner. This allows researchers to connect a system at multiple scales, providing greater simulation flexibility. Shown here are atomic point charges (top row), ion density distributions (middle), and atomic surface charge potentials (bottom). Positive potentials are shown in orange, and negative potentials are shown in blue.
(Image courtesy Song et al., design by Mike Perkins | Pacific Northwest National Laboratory)
The Science
Many important chemical processes involve reactivity and dynamics in complex solutions. Generating a fundamental understanding of reactions in solution is challenging, as current simulation techniques are limited in their ability to model the requisite large systems over long timescales. Researchers developed a new hybrid method that models part of the system at the molecular scale and other parts of the system at a larger scale, effectively connecting the two. The hybrid approach can accurately reproduce key observations of several solvent-based systems while maintaining computational efficiency compared to standard simulation approaches.
The Impact
As many chemical reactions take place in solution, researchers are focused on understanding the fundamental processes behind their reactivity and dynamics. However, the limits of established simulation methods have required compromise on either accuracy or computational efficiency. By developing a hybrid model that bridges the multiple scales involved in solvation, researchers now have enhanced flexibility and opportunities to simulate solvent systems and reactivity. Further refinements of the model will broaden the scope of its use, enabling scientists to answer questions about more solvent-based reactions accurately and efficiently.
Summary
Studies of complicated systems in solution tend to involve both large sizes and long timescales, making standard simulations such as ab initio molecular dynamics (AIMD) and even classical MD computationally expensive. One way to make these calculations cheaper is by modeling the surrounding medium in the system in less detail at the mesoscopic level. However, very few current solvation models are designed to describe interactions between the molecular scale and mesoscale. Researchers established a novel hybrid approach that couples first-principles plane-wave density functional theory with classical density functional theory (cDFT). In this approach, a region of interest described by AIMD interacts with the surrounding medium described using cDFT to arrive at a self-consistent ground state. Benchmarking against commonly used continuum models of solvation and experiments showed that the hybrid AIMD–cDFT method can produce reasonable solvation energies for a variety of molecules and ions. Researchers used the mode to examine solvent effects on a prototype SN2 reaction of the nucleophilic attack of a chloride ion on methyl chloride in the solution. The resulting reaction pathway profile and the solution phase barrier agree well with experiment results, showing that the AIMD–cDFT hybrid approach can provide insight into the specific role of the solvent on the reaction coordinate. This hybrid model overcomes the time- and length-scale constraints associated with purely ab initio methods, without sacrificing the precision of chemical interactions. The results demonstrate the potential of the hybrid approach for studying complex system geometries.
Contact
Duo Song
Pacific Northwest National Laboratory
duo.song@pnnl.gov
Kevin Rosso
Pacific Northwest National Laboratory
kevin.rosso@pnnl.gov
Funding
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, through its Geosciences Program at Pacific Northwest National Laboratory (PNNL) (FWP No. 56674). Simulations were performed using the resources of PNNL Institutional Computing (PIC) and the National Energy Research Scientific Computing Center (NERSC), a User Facility supported by the Office of Science of the U.S. DOE under Contract No. DE-AC02-05CH11231. PNNL is a multi-program national laboratory operated by Battelle Memorial Institute under Contract No. DE-AC05-76RL01830 for the DOE. We would also like to thank the NWChem and NWChemEx project team and the people that have helped in the progress of the NWChem and NWChemEx software over the years.
Published: June 3, 2024
D. Song, E. J. Bylaska, M. L. Sushko, K. M. Rosso. "Development and application of hybrid AIMD/cDFT simulations for atomic-to-mesoscale chemistry." The Journal of Chemical Physics, 160(6), 064112 (2024). [DOI: 10.1063/5.0190686]