Determining Carbonation and Critical Mineral Recovery Potential in Mafic–Ultramafic Reservoirs
New methodology can evaluate the potential for carbon storage and critical mineral recovery in undercharacterized geological settings
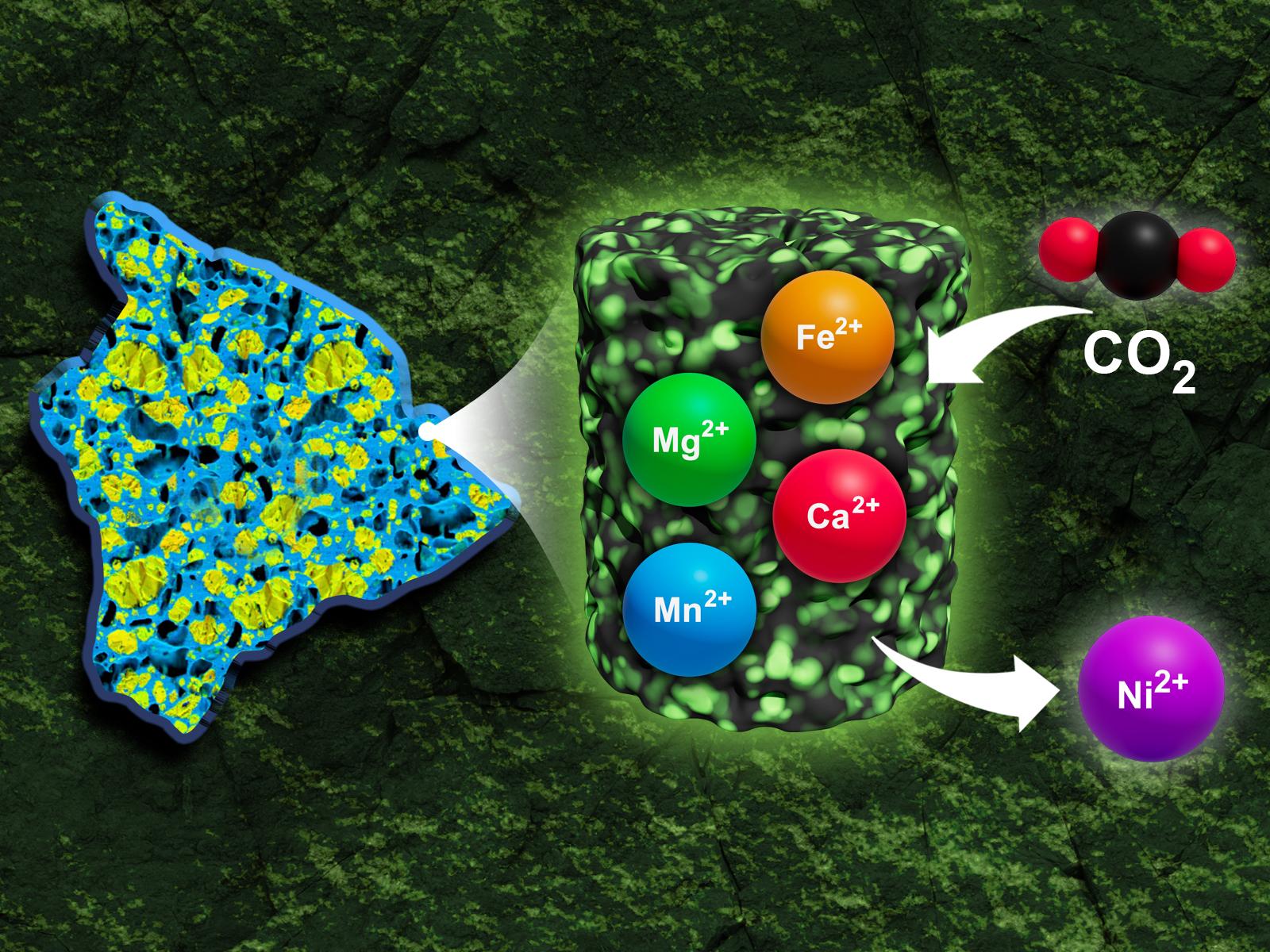
During the process of carbon mineralization, critical materials can be removed and recovered from mafic–ultramafic sites.
(Image by Mike Perkins | Pacific Northwest National Laboratory)
The Science
Increasing global emissions and warming have heightened the urgency of finding sites suitable for the long-term capture and storage of carbon dioxide (CO2). An approach that combines carbon storage with critical material extraction could make both processes more economically viable and broaden the number of suitable sites. Researchers developed a methodology to determine the potential for carbonation and critical mineral recovery in understudied mafic–ultramafic reservoirs. This method was then applied to a Hawaiian picrite (olivine-rich) basalt as a test case scenario.
The Impact
Effectively mitigating climate change requires finding ways to store CO2 permanently. Mineralization is the process of converting CO2 into stable carbonate minerals and represents one promising route to long-term carbon sequestration. Recent research has also shown that during this carbonation reaction, critical minerals can be mobilized. If collected, these minerals present an important new revenue source for carbon storage projects. By developing a methodology to assess the potential of a geological site for combined carbon storage and critical mineral recovery, this research can help identify areas that have been deemed uneconomical for traditional mining but may be suitable for carbon mineralization and critical mineral recovery.
Summary
Locating and developing practical sites for the large-scale capture and storage of CO2 has become increasingly necessary due to rising global emissions and warming. Mafic–ultramafic rocks present a unique geologic setting as they can trap injected CO2 in their pore space and mineralize it to carbonate minerals, while simultaneously releasing critical minerals. However, these reservoirs are undercharacterized relative to sedimentary carbon storage settings. Researchers developed a method for determining carbonation and critical mineral recovery potential in mafic–ultramafic reservoirs. They began with a test case—an olivine-rich basalt from the island of Hawai’i. Through various X-ray based analyses, they identified its chemistry, mineralogy, and pore network architecture. They quantified the total storage potential of the basalt as well as its realistic potential for storing CO2 permanently in the form of carbonate minerals. Based on the identified chemistry and mineralogy, the team estimated maximum and realistic mineralization and critical mineral recovery potential for the system. This storage resource estimate methodology can help accelerate the global commercialization of geologic carbon storage and critical mineral recovery.
Contact
Quin Miller, Pacific Northwest National Laboratory, quin.miller@pnnl.gov
C. Heath Stanfield, Pacific Northwest National Laboratory, charles.stanfield@pnnl.gov
Funding
This work was performed at the Pacific Northwest National Laboratory (PNNL) with support from Darin Damiani from the Department of Energy and the DOE Office of Fossil Energy and Carbon Management through its Carbon Storage program. We also acknowledge support from Dr. Douglas Wicks (DOE HQ) and the DOE Advanced Research Projects Agency-Energy (ARPA-E) MINER program under contract no. 22/CJ000/09/02. C.H.S. was supported by the DOE, Office of Science (SC), Office of Workforce Development for Teachers and Students, under the Science Undergraduate Laboratory Internships Program at PNNL. C.H.S., Q.R.S.M., R.C., and H.T.S. were partially supported by the Carbon Utilization and Storage Partnership project. A portion of this research was performed at the Environmental Molecular Sciences Laboratory, a DOE SC user facility sponsored by the Biological and Environmental Research program. PNNL is operated by Battelle for DOE under contract no. DE-AC05-76RLO1830.
Published: November 8, 2024
Stanfield, C. H., et al. 2024. “Carbon Mineralization and Critical Mineral Resource Evaluation Pathways for Mafic–Ultramafic Assets.” ACS Earth Space Chem., 8, 6, 1204–1213. [DOI: 10.1021/acsearthspacechem.4c00005]