Crystallization of Calcium Carbonate Via a Dense Liquid Phase
Calcium carbonate mineralization occurs through a multi-step process that begins with a dense liquid phase
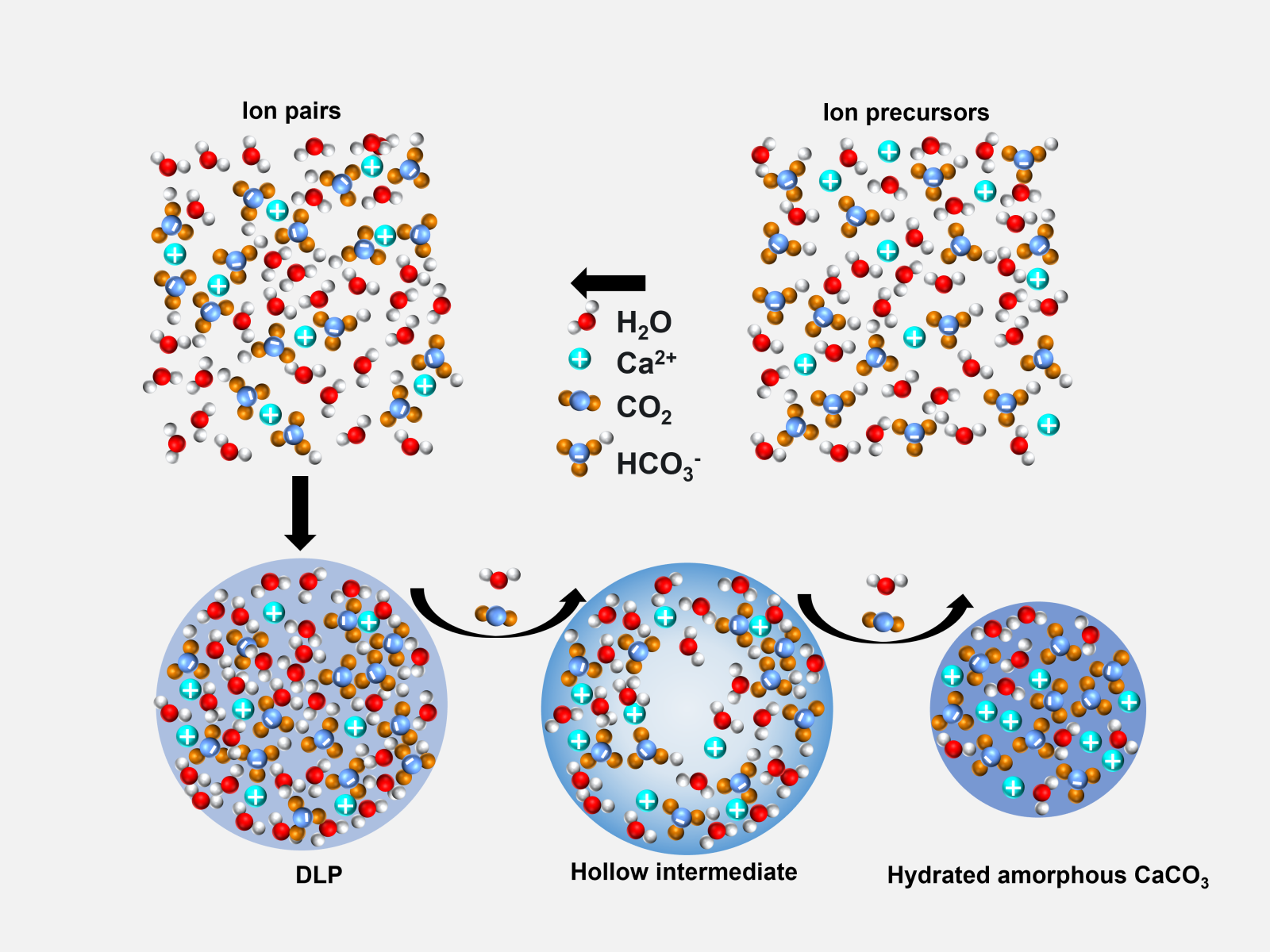
Researchers mapped the path of calcium carbonate particle formation from ion precursors, through multiple intermediates.
(Image by Ying Chen | Pacific Northwest National Laboratory)
The Science
Key to crystallization, understanding nucleation is essential to creating effective synthetic routes to materials and materials. While classical nucleation theory is accurate for some systems, many processes of interest involve complexity not integrated into classical approaches. Researchers studied solutions of highly concentrated calcium carbonate. Through a multi-pronged experimental approach, they directly visualized the formation of a dense liquid phase (DLP) and its conversion to calcium carbonate particles. The DLP first converts to a hollow particle, which then transforms into solid particles as carbon dioxide and water release from the system.
The Impact
Carbonate mineralization has gathered increasing research interest as a potential path to stably store some of humanity’s carbon dioxide (CO2) emissions. Understanding how carbonate mineralization operates at a fundamental level will allow scientists to control and direct it in carbon storage systems. This work provides insight into the nucleation process that leads to carbonate particles. It gives important information about nucleation that begins with liquid-liquid separation and describes a commonly proposed pathway of biomineralization.
Summary
Nucleation is a central process of crystallization and predicting its progression is a key challenge in materials science, geosciences, and biology. Classical nucleation theory fails to explain nucleation in a growing list of systems due to unconsidered energetic complexities. When systems are driven far from equilibrium, high chemical potentials open pathways through metastable states. These states include DLPs that form through spontaneous liquid-liquid phase separation (LLPS) without the energy barrier normally opposing nucleation. Calcium carbonate, a canonical model system for understanding crystallization in aqueous media, forms a DLP at high concentrations. However, little is known about the processes of DLP formation or solidification. Biogenically formed carbonate mineral deposits comprise the largest terrestrial reservoir of CO2 and using carbonate mineralization to capture anthropogenic CO2 emissions increases the importance of understanding these processes. In situ transmission electron microscopy, nuclear magnetic resonance spectroscopy, and infrared spectroscopy show that high-concentration calcium carbonate solutions give rise to a highly hydrated bicarbonate DLP, which forms by LLPS and transforms into hollow hydrated amorphous CaCO₃ (ACC) particles. These core-shell particles evolve into solid ACC particles before replacement by a crystalline polymorph. Acidic proteins and polymers greatly extend DLP lifetimes but leave the physical and chemical pathways seen in the additive-free system unchanged. Molecular simulations suggest that the DLP forms through direct condensation of solvated Ca²⁺⋅(HCO₃⁻)₂ complexes that react to form ACC due to proximity effects in the DLP droplets. These findings provide insight into LLPS-mediated CaCO₃ nucleation, advancing the ability to direct carbonate mineralization and elucidating an often-proposed complex pathway of biomineralization.
Contact
Jim De Yoreo, Pacific Northwest National Laboratory, James.DeYoreo@pnnl.gov
Funding
In situ LP-TEM, ATR-FTIR spectroscopy, NMR experiments, development of DFT-derived potentials, and simulations protocols for the dense liquid phase were performed at Pacific Northwest National Laboratory (PNNL) with support from the Department of Energy (DOE), Office of Basic Energy Sciences (BES), Materials Science and Engineering Division under Award FWP67554. Numerical titrations and analysis of DLP stability and properties were performed at PNNL with support from the DOE BES Division of Chemical Sciences, Geosciences, and Biosciences under award FWP16249. De novo design and synthesis of DHR proteins was performed at the University of Washington with support from the Center for the Science of Synthesis Across Scales as part of the DOE BES Energy Frontier Research Center program under Award DE-SC0019288. In situ LP-TEM, ATR-FTIR, and NMR experiments were performed under user proposals 60243 and 50824 at the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at PNNL. PNNL is a multiprogram national laboratory operated for DOE by Battelle under Contract No. DE-AC05-76RL01830. This research used the National Energy Research Scientific Computing Center resources, a DOE SC user facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231.
Published: December 5, 2024
Jin B., Y. Chen, H. Pyles, M. D. Baer, B. A. Legg, Z. Wang, N. M. Washton, K. T. Mueller, D. Baker, G. K. Schenter, C. J. Mundy, J. J. De Yoreo. 2024. “Formation, Chemical Evolution, and Solidification of the Dense Liquid Phase of Calcium (Bi)Carbonate,” Nature Mater. [DOI: 10.1038/s41563-024-02025-5]