Creating A New Paradigm for Investigating Inhomogeneous Molecular Liquids
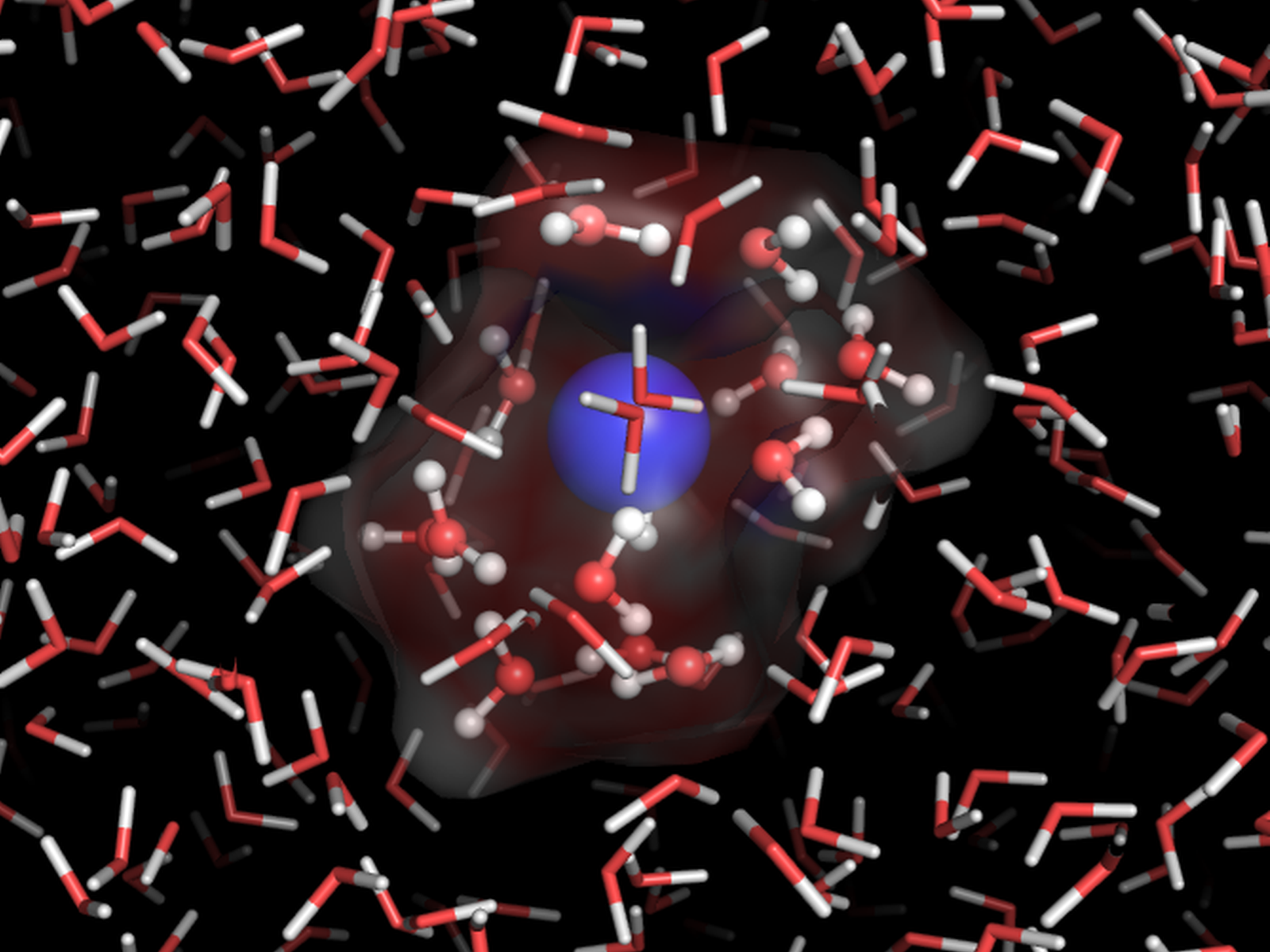
Inhomogeneities from an ion (blue sphere) interacting with water presents a challenge for prediction of macroscopic properties.
(Image courtesy of Dr. Marat Valiev | Pacific Northwest National Laboratory )
The Science
A “molecular liquid” is a collection of molecules that interact with each other to form a liquid. Water is one of the simplest examples. To understand the connection between the properties of a liquid’s molecular constituents with the observed behavior of the liquid at larger, “macroscopic” scales, consistency between theoretical descriptions must be established. This presents a significant challenge to conventional computational analysis. The work here describes a new perspective for the simulation of molecular liquids by building on past methodology to combine theoretical models for each scale. This consistency enables the accurate prediction of the properties of molecular liquids in a fraction of the time required by conventional methods.
The Impact
Molecular liquids constitute an important class of chemical systems that control the behavior of many natural processes. As such, a fundamental understanding of these systems carries an enormous practical impact toward the rational control of transformation processes in chemistry and biology. Despite these systems playing a prominent role in a majority of chemical transformations, they remain exceedingly difficult to analyze via theoretical and modeling approaches due to the complexities influencing their behavior on macroscopic scales. This work provides a new methodology for the study of molecular liquids that enhances the accuracy of thermodynamic calculations while reducing their computational cost compared to conventional simulation techniques.
Summary
The words “molecular” and “liquid” aptly define the essential nature of molecular liquids as a collection of molecules that interact with each other forming a liquid. The details of these interactions depend strongly on the nature of the molecular species, and the sheer variety of the latter underlines the rich complexity of these systems. In this study, scientists developed a new methodology for the simulation and study of inhomogeneous molecular liquids using cluster site density functional theory.
This work builds upon previous ideas of using site density functional theory (SDFT) as a fundamental framework for studying the statistical mechanics of liquids at the molecular level and demonstrates the critical importance of properly describing chemical bonds to avoid existing artifacts in conventional SDFT. By combining microscopic molecular-level descriptions with behavior of the liquid at the macroscopic scale, researchers established a systematic and practical procedure that significantly expands the scope and applicability of SDFT methods to molecular liquids.
Contact
Gregory Schenter
Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory
greg.schenter@pnnl.gov
Marat Valiev
Physical and Computational Sciences Directorate, Pacific Northwest National Laboratory
marat.valiev@pnnl.gov
Funding
- U.S. Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences.
Published: April 20, 2021
Gennady N. Chuev, Marina V. Fedotova, and Marat Valiev. 2020. “Chemical bond effects in classical site density functional theory of inhomogeneous molecular liquids” J. Chem. Phys. 152, 041101.DOI: 10.1063/1.5139619