Controlling Hierarchical Structures via Competitive Ion Adsorption
Anisotropies in surface potential driven by competitive ion adsorption can alter facet selectivity during oriented attachment
Changes in ion adsorption shift the attachment preferences of platinum nanocrystals undergoing oriented attachment.
(Image by Dongsheng Li | Pacific Northwest National Laboratory)
The Science
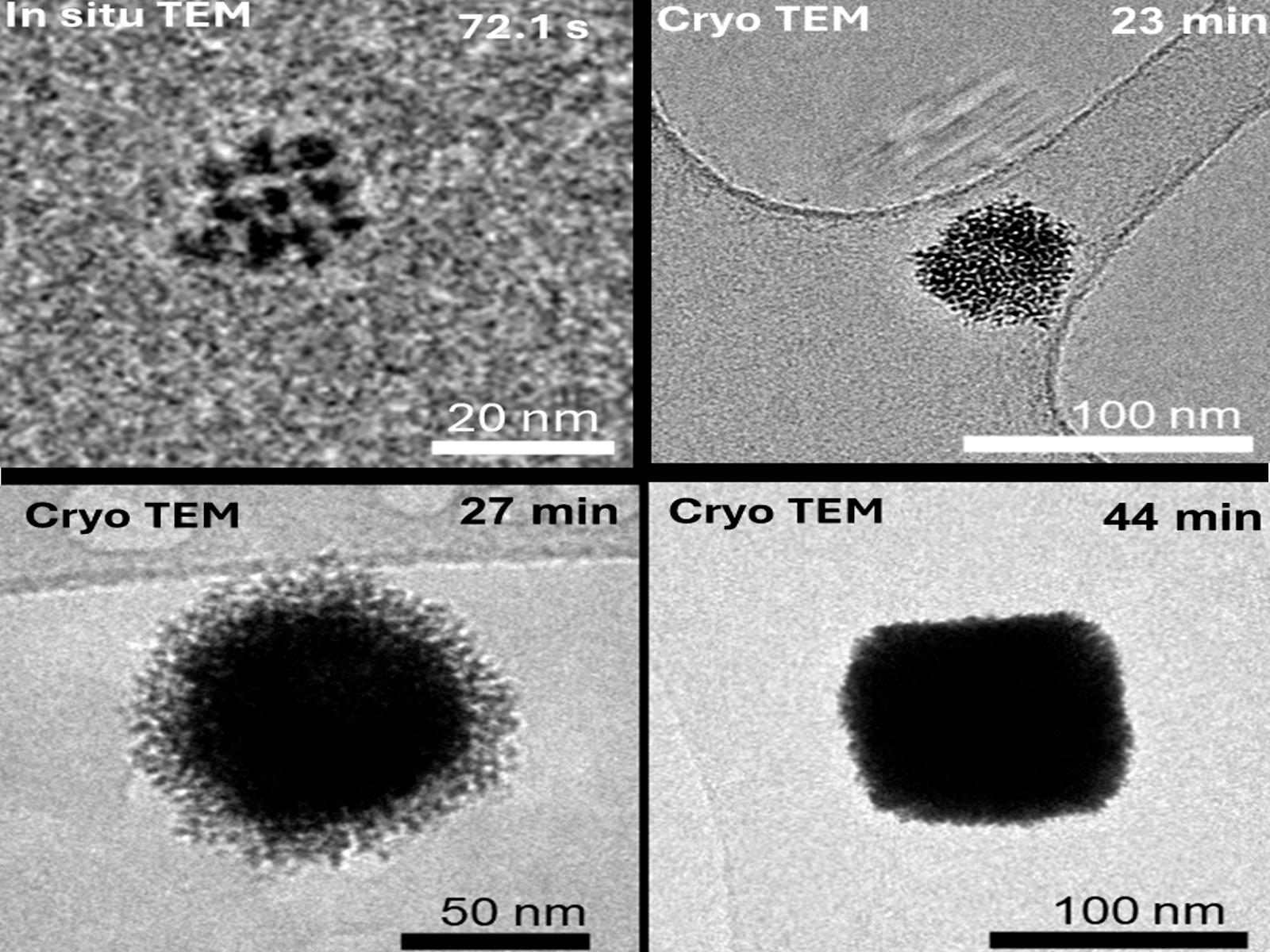
When nanoparticles assemble into larger, more complex structures, the new architecture strongly influences the overall properties of the material. Finding ways to specifically direct and tune assembly can lead to materials with desired structures and properties. Researchers studied the oriented attachment (OA) of platinum (Pt) nanocrystals into branched cubic mesocrystals. They found that over time, changes in ion adsorption shift the attachment preferences of the nanocrystals from the {100} facets to the {111} facets, leading to the initial formation of a well-defined core followed by the growth of branches on top of the core.
The Impact
Developing nanoscale materials with complex structures that can be linked to targeted functions requires discovering a wide range of synthetic routes and understanding the mechanisms of their growth pathways. OA offers an important route to creating larger structures with single-crystal-like behaviors, but there are gaps in scientific understanding of how OA enables facet selectivity. This work highlights the roles that surface electrostatic potential and electrostatic torque play during attachment, enabling researchers to manipulate the final structure of Pt materials. This strategy can be applied beyond Pt to other OA-based systems, presenting insights for designing materials with specific structures and properties.
Summary
Creating complex nanostructured materials through OA necessitates manipulating interparticle forces. These forces depend strongly on surface chemistry, such as composition, coverage, and structure, and can be modified through the effects of solution environment on interfacial chemistry. Researchers have shown that time-dependent anisotropies in surface potential driven by competitive ion adsorption can alter facet selectivity during OA. This phenomenon enables the synthesis of branched cubic Pt mesocrystals. Initially, the Pt nanoparticles attach preferentially at their {100} facets to form a well-defined cubic core. Over time, changes in ion adsorption shift the attachment preference to the {111} facets, promoting branch formation. In both stages, anisotropic surface potentials generate electrostatic torques that align the particles prior to attachment. These findings demonstrate a generalizable strategy for directing the architecture of nanomaterials through time-resolved control of interfacial chemistry during OA, offering new pathways for the design of complex structures.
Contact
Dongsheng Li, Pacific Northwest National Laboratory, Dongsheng.Li2@pnnl.gov
Jim De Yoreo, Pacific Northwest National Laboratory, James.DeYoreo@pnnl.gov
Funding
This research was performed at Pacific Northwest National Laboratory and was initially supported by the U.S. Department of Energy (DOE) Office of Science, Basic Energy Sciences (BES), Division of Materials Sciences and Engineering (MSE), Synthesis and Processing Sciences (SPS) program under Early Career award FWP 67037, before transitioning to award FWP 67554. Density functional theory calculations were funded by DOE BES MSE SPS Grant DE-FG02-07ER46414. Part of this work was performed on a project award (60753) from the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility. The density functional theory calculations used Bridges-2 at the Pittsburgh Supercomputing Center through allocation DMR110061 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296. Assistance with the surface analysis was supported by the BES under Contract No. DEAC02-06CH11357 (H. Zhou).
Published: February 20, 2026
Bae, Y., E. M. Kim, J. Chun, Z. Zhu, T. H. Moser, H. Zhang, J. Heo, Y. K. Shin, H. Zhou, J. E. Evans, E. C. S. Jensen, K. S. Mølhave, K. A. Fichthorn, J. J. De Yoreo, D. Li. “Anisotropic surface potentials induced by competitive ion adsorption enable the synthesis of branched cubic Pt mesocrystals” Nature Communications, 16, 9758 (2025). [DOI: 10.1038/s41467-025-64494-9]