Characterizing Electron Redistribution in a Key Nitrogenase Catalytic Intermediate
Characterizing the electron redistribution that allows the binding of molecular nitrogen to the nitrogenase catalytic core
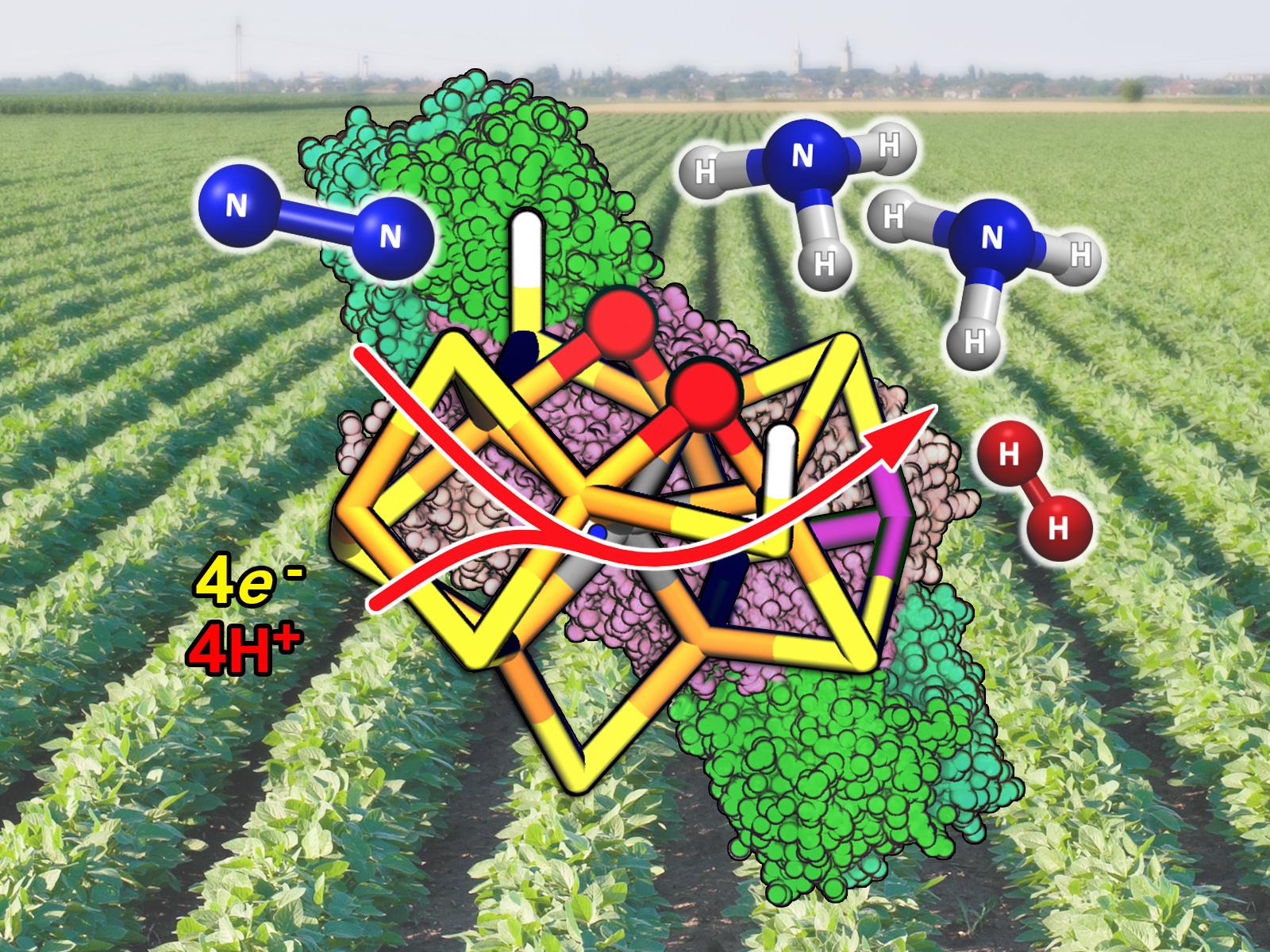
Researchers integrated spectroscopic results and computational chemistry to overcome their individual limitations and identify the electron reorganization that precedes activation of molecular nitrogen in a critical catalytic intermediate of nitrogenase.
(Image courtesy of Simone Raugei | Pacific Northwest National Laboratory)
The Science
Nitrogenase, a major enzymatic component of the biological nitrogen cycle, provides the bioavailable nitrogen that supports more than half of the human population. Despite decades of active research, the mechanism for how nitrogenase breaks up highly inert molecular nitrogen remains incompletely understood. Researchers adopted an iterative procedure that used computational chemistry and experimental information to define a simple, yet robust, model that correlates changes in electron density in the active-site metal cluster with the structure of the Janus hydride intermediate, the central catalytic intermediate of nitrogenase. Understanding the electron distribution changes in the enzyme catalytic core prior to molecular nitrogen binding represents an important step toward understanding how this vital enzyme works.
The Impact
Ammonia, the primary ingredient in nitrogen-based fertilizers necessary for crop production, is currently industrially produced from fossil fuels using the celebrated and energy-intensive Haber-Bosch process. Creating new nitrogenase-inspired catalysts requires developing a fundamental molecular understanding of the nitrogen (N2) to ammonia conversion mechanism. After identifying the nature of the Janus intermediate, research on nitrogenase can focus on how the enzyme breaks the strong molecular nitrogen triple bond and forms ammonia. That information can then serve as a guideline to create more efficient and environmentally friendly catalysts.
Summary
Our society uses a great deal of ammonia—most of it for fertilizers required to grow our food. Currently, most ammonia is made in large industrial plants from atmospheric N2 and fossil fuels, accounting for about 2 percent of global energy consumption. Nature uses an enzyme called nitrogenase to perform this same chemistry in a more environmentally benign way. Understanding the molecular details of the catalytic core of the enzyme and the key intermediate step in this chemical conversion is critical to creating an industrially useful mimic for ammonia production. Adopting an iterative procedure, researchers used computational chemistry and experimental information to define a simple, yet robust, model to correlate changes in the electron density of the active-site metallocofactor with the structure of the catalytically central Janus hydride intermediate. With the nature of the Janus intermediate identified, research on nitrogenase can progress to the next level and focus on how the enzyme breaks the strong molecular N2 triple bond and forms ammonia.
The experimental work was performed at Northwestern University and Utah State University, while the computational work was performed using supercomputers at both the National Energy Research Scientific Computing Center and Environmental Molecular Sciences Laboratory.
PNNL Contact
Simone Raugei, Pacific Northwest National Laboratory, Simone.Raugei@pnnl.gov
Funding
The U.S. Department of Energy, Office of Science, Basic Energy Sciences funded this work; the Division of Chemical Sciences, Geosciences, and Bio-Sciences Physical Biosciences program funded both the protein preparation (through award number DESC0010687 to L.C.S), and the calculations (through award number DEAC05-76RL01830/FWP66476 to S.R.). Portions of the work were also supported by the National Institutes of Health and the National Science Foundation.
Published: April 6, 2021
D. A. Lukoyanov, Z.-Y. Yang, D. R. Dean, L. C. Seefeldt, S. Raugei, and B. M. Hoffman. “Electron Redistribution within the Nitrogenase Active Site FeMo-cofactor During Reductive Elimination of H2 to Achieve N≡N Triple-Bond Activation” J. Am. Chem. Soc. 124, 21679-21690. (2020)