Tracking Solvent Motions Coupled to Electron Transfer
Unraveling the complex processes involved in this critical reaction step
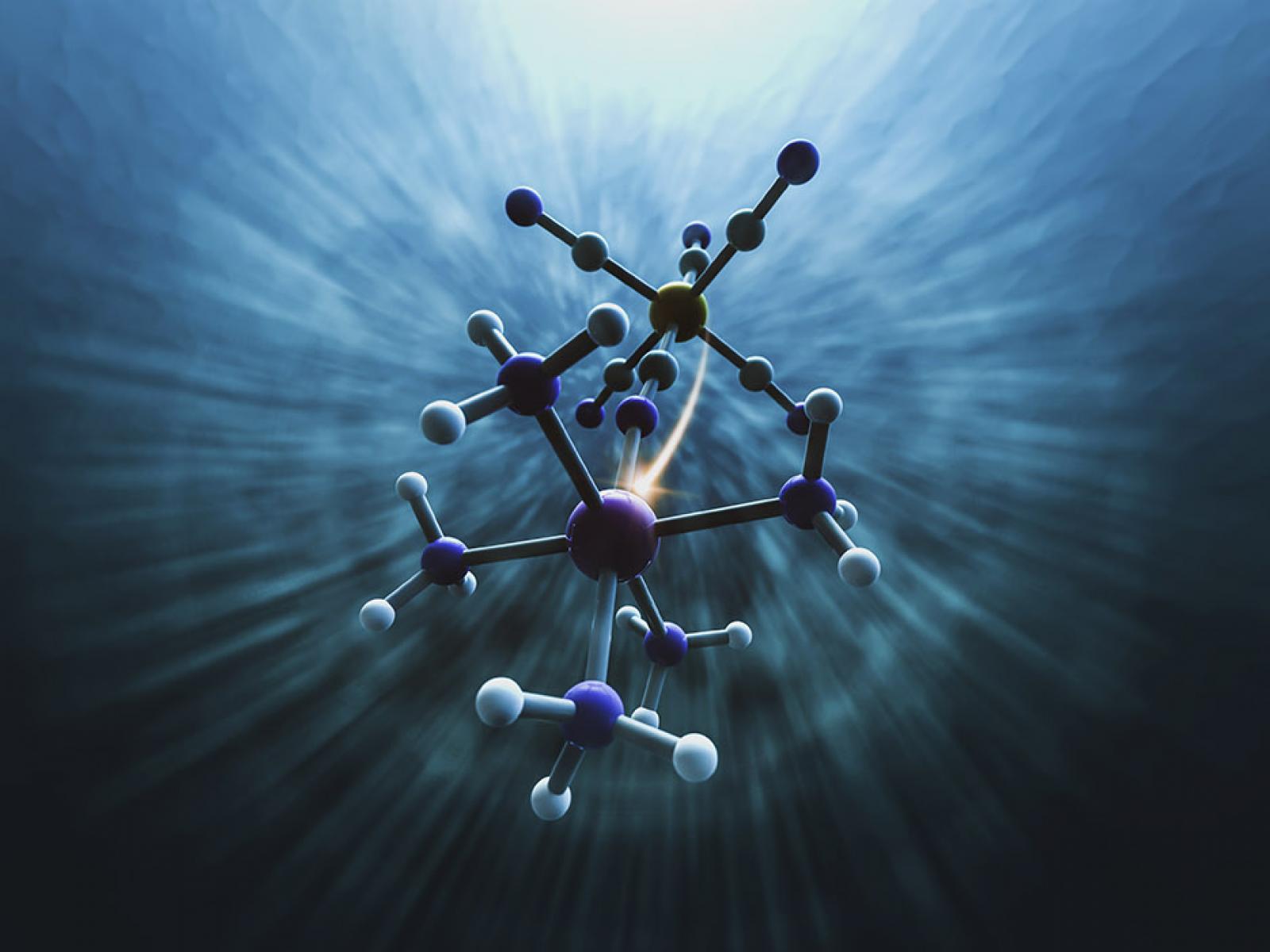
Depiction of the electron transfer between iron and ruthenium atoms in water.
(Image by Greg Stewart | SLAC National Accelerator Laboratory)
A team of researchers from the University of Washington, led by Munira Khalil, partnered with Pacific Northwest National Laboratory (PNNL) physicist Niri Govind, Environmental Molecular Sciences Laboratory computational scientist Amity Andersen, and scientists from SLAC National Accelerator Laboratory to investigate the effects of solvent molecules on electron transfer. By combining state-of-the-art computational and experimental approaches, these researchers have begun to unravel the complex processes involved in this critical reaction step.
“Our work sheds light on the molecular details of charge flow within a molecule, coupled to the motions of the surrounding water molecules in real-time with atomic scale resolution,” said Govind. Their results were published in Nature Chemistry.
Electron transfer reactions are crucial for the development of next-generation molecules and materials for artificial light-harvesting, photochemical energy conversion, and photocatalysis. They also play a vital role in biological systems, such as photosynthesis. These reactions occur on ultrafast timescales. They are typically measured in femtoseconds, which represent one millionth of one billionth of a second, making them difficult to study experimentally.
Researchers used a combination of femtosecond X-ray solution scattering and X-ray emission spectroscopy to study electron transfer in a molecular complex containing iron and ruthenium atoms in water. Govind and Andersen simulated the electron transfer process, reorganization of water molecules, and X-ray signatures with detailed ground and excited-state calculations to interpret the experimental data. The simulations were performed with NWChem, an open-source PNNL-developed computational chemistry program using computing resources provided by the Environmental Molecular Sciences Laboratory a U.S. Department of Energy Office of Science user facility at PNNL.
These findings can be applied to both natural and artificial energy conversion mechanisms and may ultimately shape the development of clean energy technologies.
Published: April 15, 2021