Detecting Underscreening and Generalized Kirkwood Transitions in Aqueous Electrolytes
Combining experiments and theory to predict the collective properties of electrolyte solutions
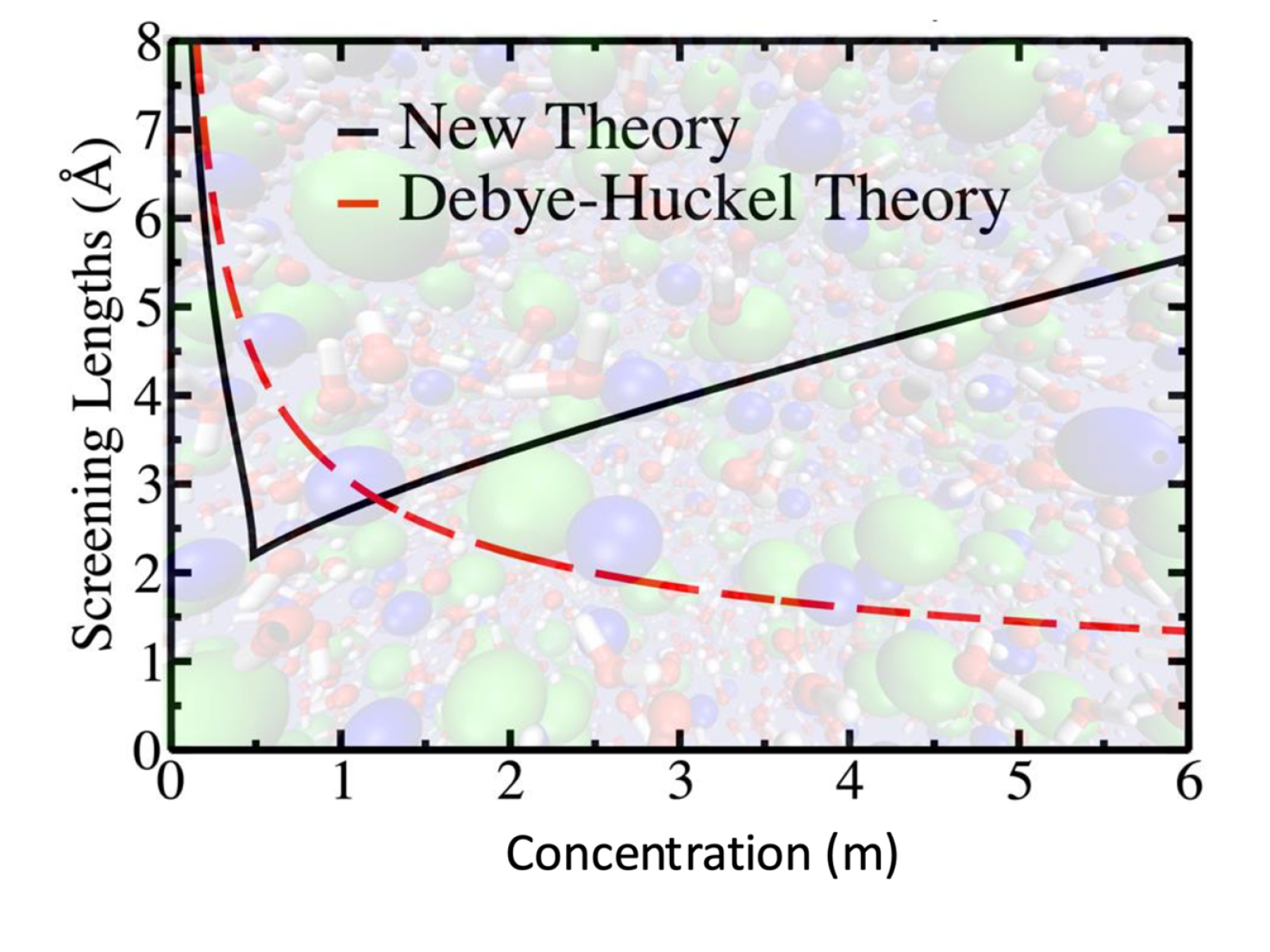
A newly developed, generalized theory can improve scientific understanding of electrolyte solutions by considering short-range effects and their subtle interplay with long-range phenomena.
(Image by Hadi Dinpajooh, Shawn Kathmann, Gregory Schenter, and Chris Mundy | Pacific Northwest National Laboratory)
The Science
Electrolyte solutions are important to numerous applications, from the synthesis of materials to the workings of energy storage devices. Researchers worked to develop an understanding of how these solutions of charged ions collectively behave across a wide range of concentrations. They quantified the breakdown of the traditional Debye-Hückel theory in concentrated electrolytes, identifying the emergence of underscreening phenomena and its connections to Kirkwood Transitions, where charge screening follows a periodic pattern. They established a rigorous connection between the measured small angle X-ray scattering signal and the charge-charge correlations responsible for the Kirkwood Transitions.
The Impact
Ion correlation leads to phenomena in aqueous solutions ranging from nucleation to self-assembly by controlling the strength of electrostatic forces between objects and charges. By providing new insights for quantifying ion correlations across a wide range of electrolyte concentrations, this work can lead to more accurate representations of screening and similar phenomena in models. From an experimental side, this increased knowledge can aid in the synthesis, design, and assembly of materials.
Summary
Collective properties of electrolyte solutions play an important role in controlling their overall behavior. In particular, ion correlation influences the distribution and motion of the electrolyte. While Debye-Hückel theory accurately describes ion correlation for dilute electrolytes, it breaks down for high ion concentrations. Researchers combined experimental results with new theoretical approaches to develop a general way to represent ion correlation in concentrated electrolytes. They identified the prepeak region in small angle X-ray scattering measurements to directly access information about charge-charge correlation and screening. This data can provide screening lengths in electrolyte solutions, which is crucial for modulating self-assembly, clustering, and nucleation phenomena. The team also developed a theory that includes coupling of both charge and density in electrolyte solutions. This new generalized theory can more accurately explain and predict experimental results than classical theories of electrolytes. This work is important to the development of electrolyte-based technologies and is helping build a more complete picture of electrolyte behavior across all ion concentrations.
Contact
Hadi Dinpajooh, Pacific Northwest National Laboratory, hadi.dinpajooh@pnnl.gov
Greg Schenter, Pacific Northwest National Laboratory, greg.schenter@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Condensed Phase and Interfacial Molecular Science programs FWP 16249 and FWP 16248. Part of the computational resources are provided by National Energy Research Scientific Computing Center, a Department of Energy Office of Science user facility using NERSC Award No. BES-ERCAP-26557.
Published: June 6, 2025
Dinpajooh M., E. Biasin, E.T. Nienhuis-Marcial, S.T. Mergelsberg, C.J. Benmore, G.K. Schenter, and J.L. Fulton, et al. 2024. "Detecting Underscreening and Generalized Kirkwood Transitions in Aqueous Electrolytes." Journal of Chemical Physics 161, no. 15:151102. [doi:10.1063/5.0234518]
Dinpajooh M., N.N. Intan, T. Tim Duignan, E. Biasin, J.L. Fulton, S.M. Kathmann, and G.K. Schenter, et al. 2024. "Beyond the Debye-Hückel Limit: Towards a General Theory for Concentrated Electrolytes." Journal of Chemical Physics 161, no. 23:230901. [doi:10.1063/5.0238708]