Using Zeolite Scaffolds to Create Better Catalysts
Mo4S4 clusters encaged in zeolites mimic the FeMo-cofactor of nitrogenase
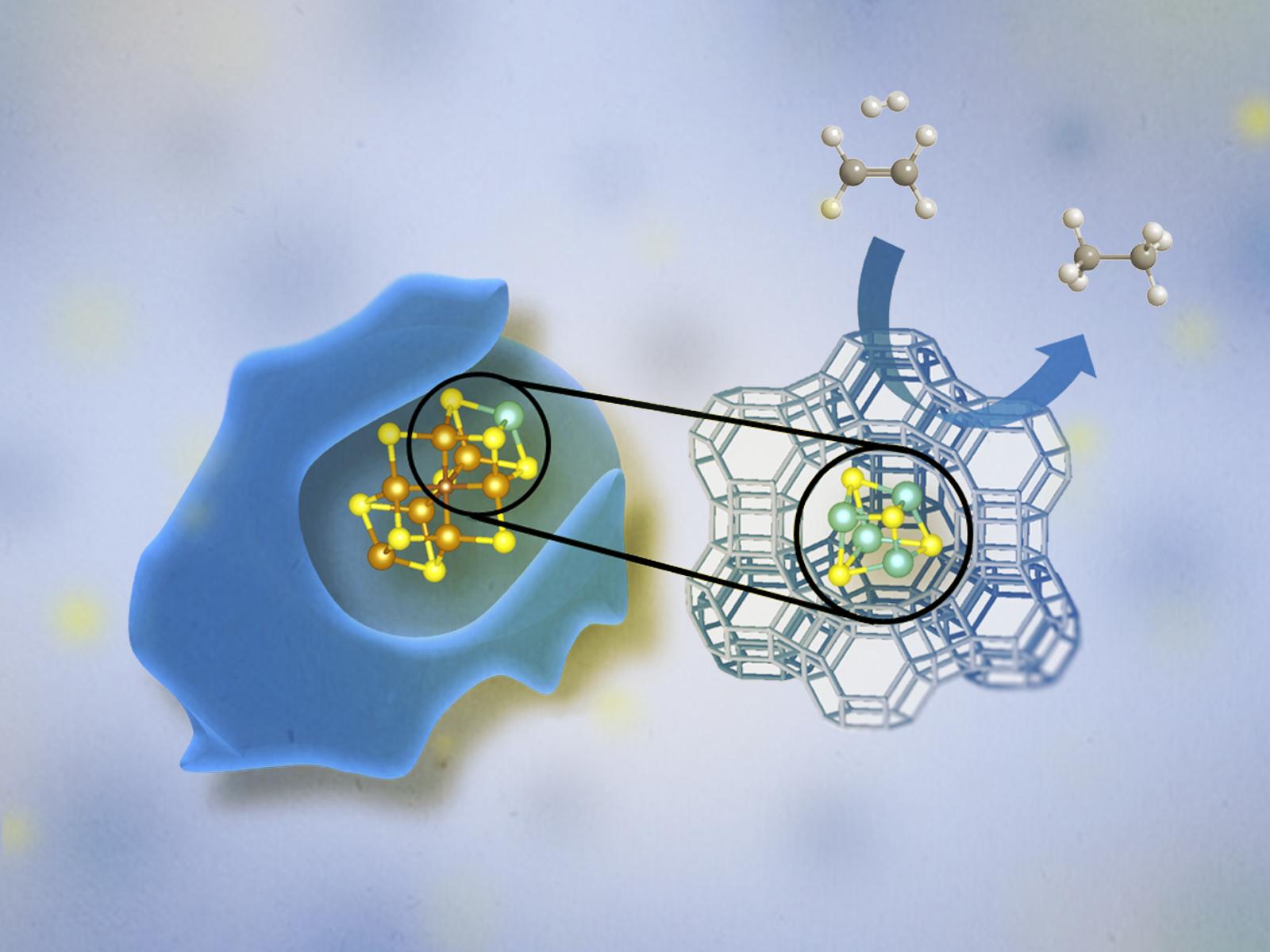
A tetranuclear (Mo4S4) molybdenum sulfide cluster in the pores of a zeolite resembles a nitrogenase Fe-Mo cofactor in enzymes. The cluster is stable and active for the hydrogenation of small olefins.
(Illustration by Stephanie King | Pacific Northwest National Laboratory)
The Science
Transition metal sulfide (TMS) catalysts greatly interest the research community due to their ability to catalyze reactions for important industrial applications, such as the hydrotreatment of refinery fractions and renewable feedstocks. In this study, researchers synthesized dinuclear (Mo2S4) and tetranuclear (Mo4S4) molybdenum sulfide TMS clusters within the large internal cavities, called supercages, of a faujasite zeolite to form a structure similar to the FeMo-cofactor of a nitrogenase enzyme. Both synthesized di- and tetra-nuclear structures contain unpaired electrons, just as the nitrogenase FeMo-cofactor does, and show identical catalytic activity per cluster. This research resolves previous uncertainties in TMS catalyst structures and provides a foundation for future TMS synthesis studies.
The Impact
The robust scaffolding afforded by the zeolite stabilizes the molybdenum sulfide clusters and allows them to carry out ethene hydrogenation over long periods of time, while layered MoS2 deactivates significantly under the same conditions. The enhanced stability and activity of this bio-inspired catalyst compared to conventional catalysts offers a new opportunity for improving industrial hydrotreatment processes. Additionally, this class of robust, bio-inspired catalysts will create exciting opportunities regarding the chemical and structural variability of future catalysts.
Summary
TMS catalysts have many interesting commercial applications, thus improving their stability, functionality, or method of synthesis would be greatly beneficial to many industries. In this work, stable bio-inspired TMS catalysts were synthesized using chemical vapor deposition to incorporate molybdenum sulfide cluster into the supercages of faujasite zeolites. This produced catalysts with a similar structure to nitrogenase enzyme’s FeMo-cofactor. These structures were also able to hydrogenate olefins without the need for additional sulfur, as is necessary for many sulfide-based catalysts.
Though similar synthesis protocols have been reported, this study resolves previous ambiguities in the structural characterization using a combination of computational and experimental techniques. Thus, this research provides the basis for future experiments involving mixed metal sulfides using the same zeolite scaffolding.
Portions of the experiments were performed at the Environmental Molecular Science Laboratory, a national scientific user facility sponsored by the U.S. Department of Energy’s Biological and Environmental Research program located at Pacific Northwest National Laboratory.
Contact
Libor Kovarik
Environmental Molecular Sciences Laboratory
Libor.Kovarik@pnnl.gov
Funding
This work was funded by the Chevron Energy Technology Company. Conceptual work and structural determinations were supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences and Biosciences. The authors gratefully acknowledge the Leibniz Supercomputing Center for funding this project by providing computing time on their Linux-Cluster. The X-ray absorption and emission spectroscopy measurements were performed on beamline ID26 at the European Synchrotron Radiation Facility, Grenoble, France.
Published: May 11, 2021
R Weindl, R Khare, L Kovarik, A Jentys, K Reuter, H Shi, J A Lercher. 2021. "Zeolite-stabilized di- and tetranuclear molybdenum sulfide clusters form stable catalytic hydrogenation sites" Angew Chem Int Ed, 60. 9301-9305.
[DOI: 10.1002/anie.202015769]