Understanding the Interfacial Compatibility Between a Potential CO2 Separation Membrane and Capture Solvents
Surface characterization allows researchers to identify if solvent-membrane combinations are compatible
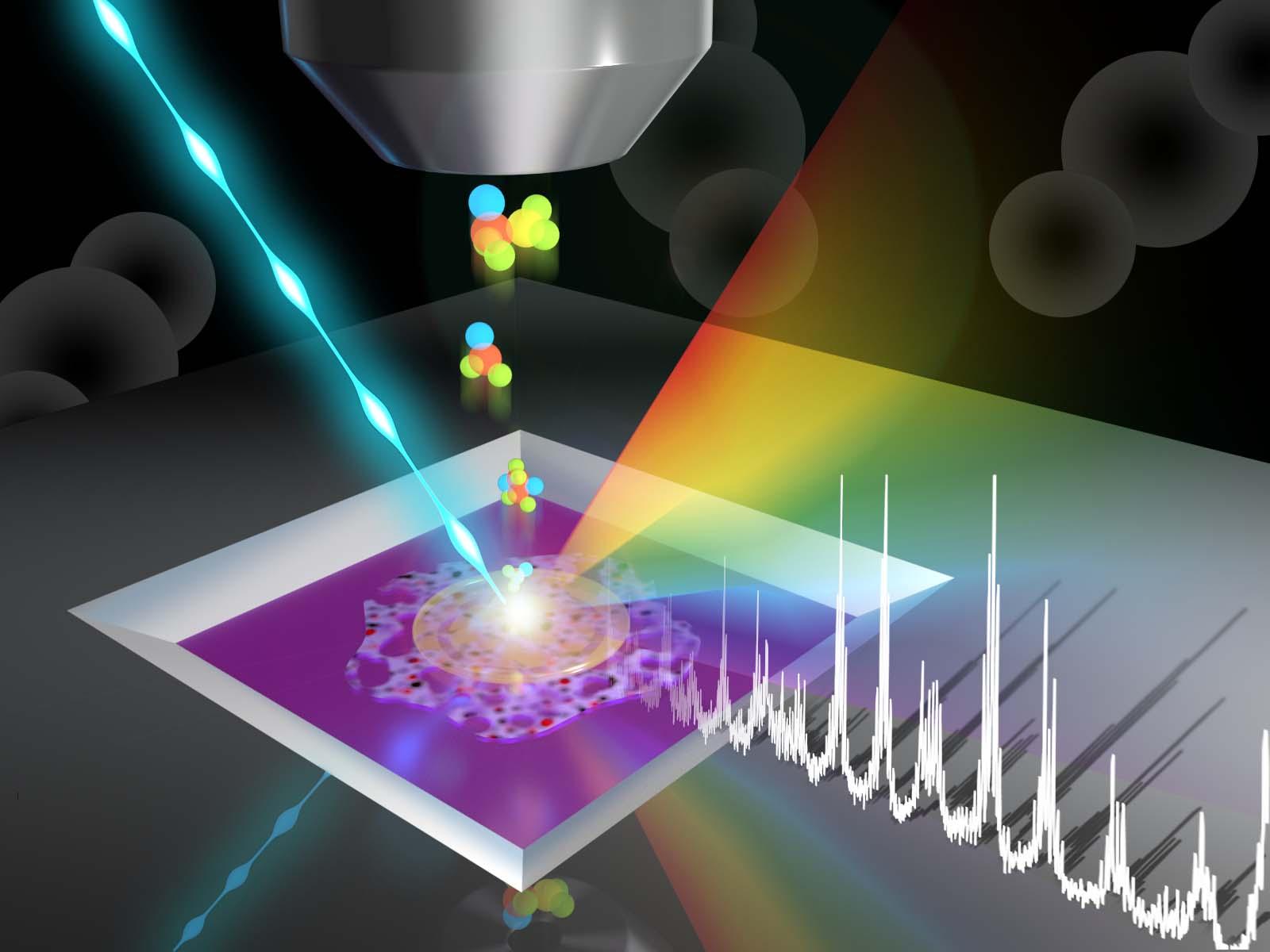
Promising initial separation membrane candidates showed chemical incompatibility with current carbon dioxide capture solvents, identifying a bottleneck for advanced direct air capture devices.
(Image by Mike Perkins | Pacific Northwest National Laboratory)
The Science
Direct air capture (DAC) of carbon dioxide (CO2) would allow humanity to remove CO2 from the air after its emission. However, doing so requires separating CO2 from a mixture of gases, including nitrogen, oxygen, and water. A collaborative team of researchers is combining CO2 capture solvents and separation membranes to enable advanced DAC devices. Creating devices requires combining compatible solvents and membranes. The team showed that current capture solvents and initial separation membrane candidates are not chemically compatible, with reactions occurring where the solvent and membrane come together. This identified an unexpected area in need of additional research.
The Impact
Producing a practical DAC device requires stability across all the component parts when combined. Characterizing the surfaces of membranes exposed to solvent, demonstrated in this work, can verify solvent-membrane compatibility. Determining which combinations are incompatible offers insights into design principles for next-generation membranes, including chemical structures to use or avoid.
Summary
DAC represents one way to move towards decarbonizing energy usage and industrial processes by separating CO2 from other gases in the air. A collaborative team of researchers is combining CO2 capture solvents and separation membranes to enable advanced DAC devices.
Initial work combining a PEEK-ionene candidate separation membrane with capture solvents produced visible changes in the membrane. Researchers employed surface-sensitive tools, including time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy to directly probe the chemical changes occurring at the membrane-solvent interface. Chemical mapping of the membrane surface showed changes attributable to reactions with the solvent. They found the best compatibility between the studied membrane and a capture solvent with a pyridine structure. This suggests that compatible membranes and solvents may have chemically similar structures. These results can help guide the development of the next generation of CO2 separation membranes and capture solvents.
PNNL Contact
Xiao-Ying Yu, Pacific Northwest National Laboratory, xiaoying.yu@pnnl.gov
Funding
This work was jointly supported by the Department of Energy, Office of Science, Basic Energy Sciences program, Divisions of Chemical Sciences, Geosciences, and Biosciences and Materials Sciences and Engineering under FWP 76830. The X-ray photoelectron spectroscopy measurements were performed at the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at Pacific Northwest National Laboratory.
Published: March 23, 2022
J. Gao, J. Son, Y. Zhang, J. Bara, K. E. O’Harra, M. Engelhard, D. Heldebrant, R. Rousseau, and X.-Y. Yu, “The interfacial compatibility between a potential CO2 separation membrane and green solvents.” Carbon Capture Science & Technology, in press, 100037 (2022). [DOI: 10.1016/j.ccst.2022.100037]