Understanding Dissolution Anisotropy in Metal Oxides
Varying environmental conditions enabled researchers to quantitatively control the dissolution of metal oxide nanorods
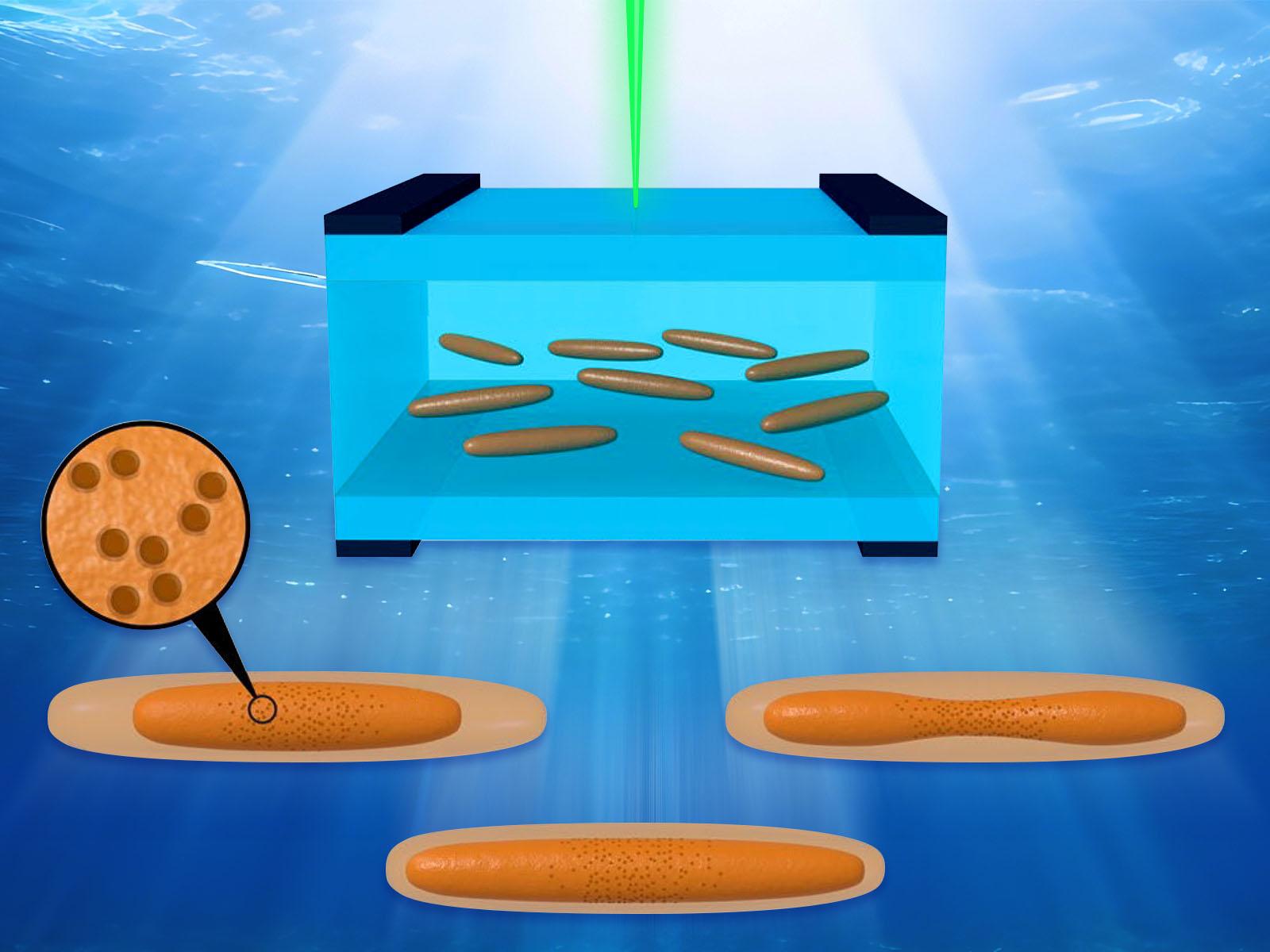
Akaganeite nanorods exhibit different dissolution mechanisms based on their surrounding environment.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Redox-active metal oxides are found throughout the environment, where their dissolution affects mineral formation and nutrient cycling. Researchers used liquid phase transmission electron microscopy (LP-TEM) to visualize the dissolution of akaganeite (β–FeOOH). They found that varying electron beam radiolysis, pH buffers, and chloride anions altered the dissolution mechanism in a predictable and tunable way. The team was then able to control the akaganeite dissolution by varying the conditions in the LP-TEM cell.
The Impact
Dissolution of redox-active metal oxides is important in the environment and technology. However, it remains poorly understood at the nanoscale, particularly when acids and bases are active. This work deepens scientific understanding of facet-dependent dissolution through acidic and reductive attacks. It provides new clues into the processes controlling the dissolution rate and morphologies of iron oxide minerals in natural systems.
Summary
Understanding how nanomaterials dissolve is essential to developing deep knowledge of biogeochemical cycles and creating new technologies. In particular, iron-based oxides are broadly available and play an important role in environmental cycling. Researchers used LP-TEM to study the dissolution mechanisms of akaganeite, an iron oxide-hydroxide mineral. The team synthesized akaganeite nanorods and fully characterized their structure and inherent defects. The nanorods were then placed into LP-TEM cells under an electron beam with a range of conditions, including different pH levels and ion species. Beam-induced radiolysis contributes to two main processes: acid-promoted dissolution and the reduction of lattice iron (III) to soluble iron (II), which drives dissolution. The presence of pH buffers offsets, and can potentially eliminate, the acid-promoted dissolution processes. Finally, chloride ions lead to a complexation-based dissolution mechanism. Identifying these different mechanisms and how they affect akaganeite nanorods provides evidence that LP-TEM can be an effective tool for identifying and understanding the mechanisms behind dissolution processes. This research revealed how aspects of the chemical environment control akaganeite dissolution, providing potential routes to tune the system.
PNNL Contact
Kevin Rosso, Pacific Northwest National Laboratory, kevin.rosso@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES) program, Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences program at Pacific Northwest National Laboratory (PNNL). The SEM, XRD, and 3D tomography STEM were performed at EMSL, the Environmental Molecular Science Laboratory, a DOE Office of Science user facility at PNNL.
Published: July 14, 2023
Liu, L., M. Sassi, X. Zhang, E. Nakouzi, L. Kovarik, S. Xue, B. Jin, K. M. Rosso, and J. J. De Yoreo. 2023. Proc. Natl. Acad. Sci. 120, e2101243120. [DOI:10.1073/pnas.2101243120]