Surface Oxygen Functionality Controls the Selective Transport of Metal Ions through Graphene Oxide Membranes
The surface oxygen functionality of graphene oxide may be tuned using ultraviolet light, affecting how differently charged ions move through the material
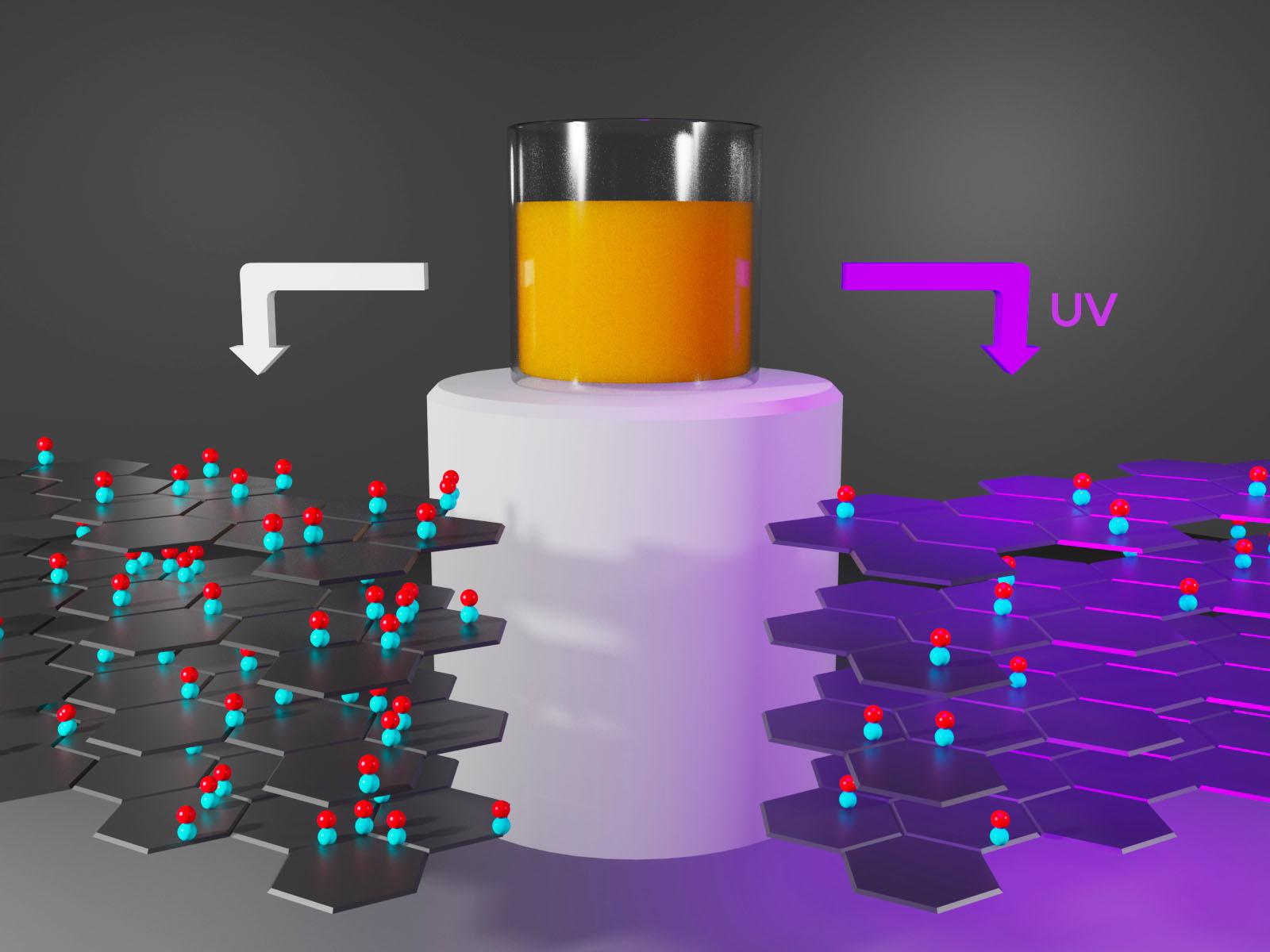
Ultraviolet light reduces graphene oxide membranes, changing the functional groups on the material surface.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Graphene oxide (GO) membranes have shown promise for separating ions from mixed solutions based on size. Research into approaches to modify the membranes for more selective separations is ongoing. Scientists discovered that reducing GO membranes with ultraviolet (UV) light alters the oxygen functional groups on the GO surface. This modification results in a different, chromatography-like separation mechanism that is selective for charge rather than size. Larger, doubly charged cations, such as calcium, move through the membranes faster than smaller, singly charged cations such as lithium.
The Impact
Developing efficient, selective, and scalable separations for critical materials, including lithium and magnesium, is essential to meeting the increasing demands for clean energy technologies and alleviating challenges with domestic supply chains. While previous work used GO membranes for size-based separations, UV light reduction expands the potential uses of GO membranes by altering the separation mechanism. This modification approach allows researchers to tune the functionality of GO membranes via a straightforward method that uses no harsh or specialty chemicals.
Summary
Previous studies indicated that GO membranes discriminate between different ions based on a size-exclusion mechanism controlled by the dimensions of the interlayer transport channels. GO reduced through exposure to ultraviolet light (UV-rGO) displays ion transport inconsistent with size exclusion. Smaller lithium cations permeate more slowly through the UV-rGO membranes than larger cations, such as calcium and magnesium, resulting in a 3–4-fold improvement in the separation selectivity between these representative cations. UV exposure selectively removed hydroxyl (–OH) groups from the GO basal planes, leading to enhanced interactions of metal cations with functional groups located at the edges of GO. This resulted in a lower ratio of free mobile lithium in solution compared to calcium cations. A separation mechanism analogous to chromatography is proposed for UV-rGO, emphasizing the crucial role of different oxygen groups on GO in controlling selective ion transport through GO membranes.
Contact
Grant Johnson, Pacific Northwest National Laboratory, Grant.Johnson@pnnl.gov
Manh-Thuong Nguyen, Pacific Northwest National Laboratory, manhthuong.nguyen@pnnl.gov
Venky Prabhakaran, Pacific Northwest National Laboratory, venky@pnnl.gov
Funding
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences program; Chemical Sciences, Geosciences, and Biosciences Division; FWP 81462 (Harnessing Confinement Effects, Stimuli, and Reactive Intermediates in Separations). PNNL is a multiprogram national laboratory operated by Battelle for the U.S. DOE under Contract DE-AC05-6RL01830. Computer resources were provided by Research Computing at PNNL and the National Energy Research Scientific Computing Center (NERSC), a U.S. DOE Office of Science User Facility operated under Contract No. DE-AC02-05CH11231.
Published: July 15, 2024
Y. Yin, et al., “Distinct ion transport behavior between graphene oxide and UV-irradiated reduced graphene oxide membranes.” Chemical Engineering Journal, 493, 152304 (2024). [DOI: 10.1016/j.cej.2024.152304]