Superlattice Engineering Offers Cation Control
Artificial structuring in carefully designed (SrNiO3)1/(LaFeO3)n superlattices stabilized Ni4+ cations that would otherwise be unstable
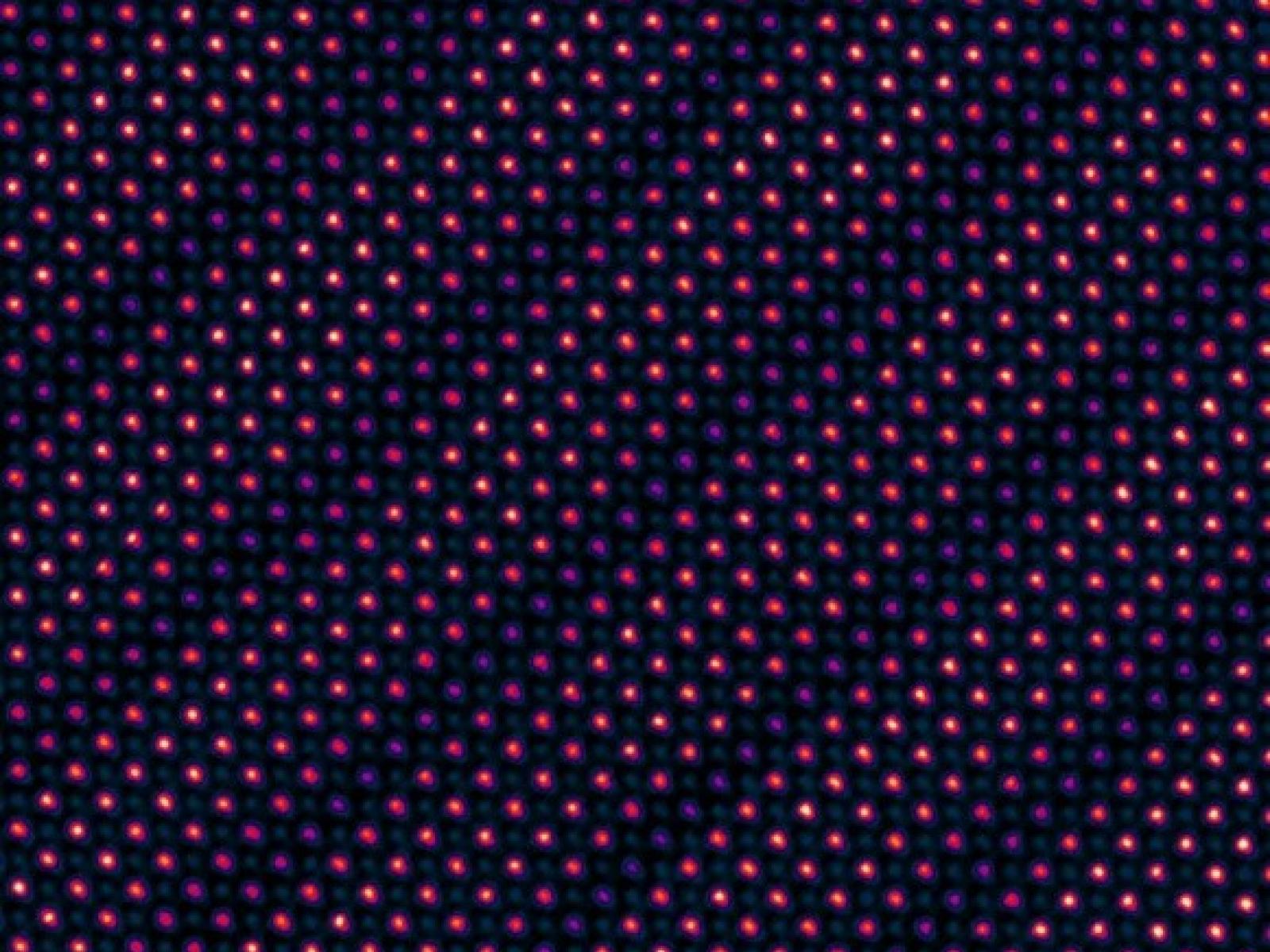
A cross-sectional scanning transmission electron micrograph of a (SrNiO3)1/(LaFeO3)n=5 superlattice reveals excellent crystallinity and precise layer control.
Image courtesy of Le Wang and Steven Spurgeon | PNNL
The Science
Complex transition metal oxides have sufficient structural flexibility to produce a wide range of functional properties for electronics and energy storage applications. To control these properties, researchers grow thin layers of these materials seeking to control their crystal structure. Controlling crystal structure opens the door for tuning electronic structure and possibly achieving novel properties. Electronic structure in oxides is largely controlled by the charges on the cations. Ni4+ ions are energetically unstable and rarely found in chemical compounds. Now a team of researchers, led by scientists from Pacific Northwest National Laboratory, have stabilized Ni4+ in a carefully designed superlattice of SrNiO3 and LaFeO3. The cubic perovskite SrNiO3, which is not observed in bulk form, was shown to form in these superlattices, but only at the thickness of one crystalline unit or unit cell.
The Impact
This work shows that accurate control of material structure through superlattice engineering may be generally useful for the synthesis and discovery of metastable, layered functional materials.
Summary
A team of researchers, led by scientists from Pacific Northwest National Laboratory, stabilized Ni4+ cations in a superlattice of SrNiO3 and LaFeO3. To do this, they deposited alternating layers of SrNiO3 and LaFeO3 with varying thicknesses for each and found that well-ordered SrNiO3 nucleated only when a single unit cell of SrNiO3 was included. By controlling the thickness of the adjacent LaFeO3 layers, they could control the charges on the Fe and Ni cations.
If a single unit cell of LaFeO3 is used, resulting in a (SrNiO3)1/(LaFeO3)1 superlattice, the Ni is trivalent (Ni3+) and the Fe is tetravalent (Fe4+). However, as the LaFeO3 layer thickness increased, the Ni becomes tetravalent (Ni4+) and the Fe trivalent (Fe4+). It was found that additional layers of LaFeO3 induce larger rotations of the FeO6 octahedral cages in the LaFeO3 layers. The result is that the structure of the LaFeO3 layers approaches that of bulk LaFeO3. This structural change modifies the densities of electronic states such that holes can localize in the SrNiO3 layers, producing Ni4+ in the (SrNiO3)1/(LaFeO3)5 superlattice.
Contact
Scott Chambers
Pacific Northwest National Laboratory
scott.chambers@pnnl.gov
Funding
This work is supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated by Argonne National Laboratory. This research was performed using the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research.
Published: October 2, 2020
Le Wang, Zhenzhong Yang, Mark E. Bowden, John W. Freeland, Peter V. Sushko, Steven R. Spurgeon, Bethany Matthews, Widitha S. Samarakoon, Hua Zhou, Zhenxing Feng, Mark H. Engelhard, Yingge Du, and Scott A. Chambers. “Hole-Trapping-Induced Stabilization of Ni4+ in SrNiO3/LaFeO3 Superlattices,” Advanced Materials (2020). [DOI: 10.1002/adma.202005003]