Solving a MOF Mystery
Revealing the kinetic controls of metal-organic framework/metal oxide heterostructure
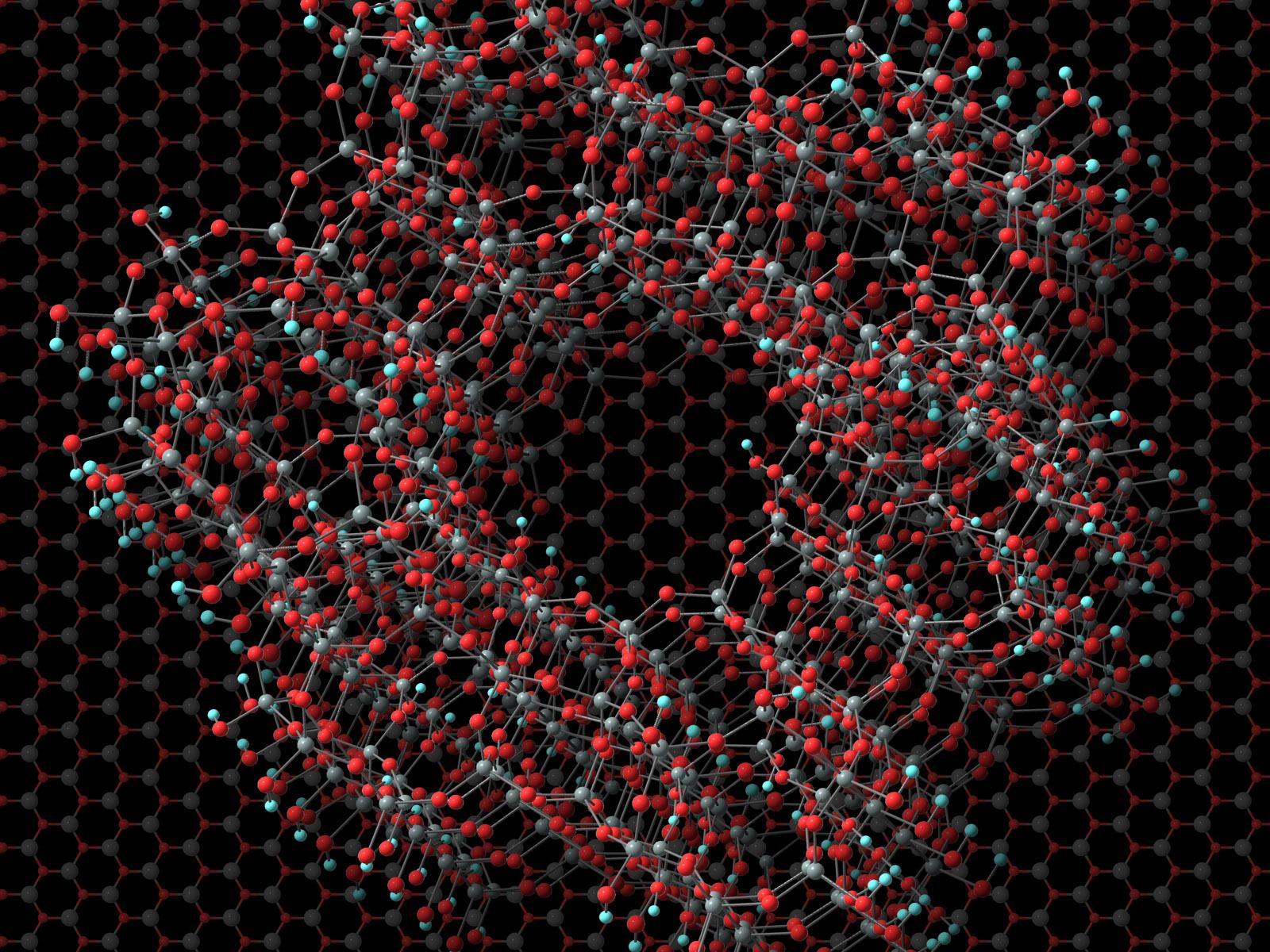
Metal-organic frameworks (MOFs)/semiconducting oxide heterostructures exhibit unique properties beyond those of individual components, but their design requires an understanding of energetic and kinetic controls at MOFs–substrate interfaces.
The Science
Metal-organic frameworks (MOFs)/semiconducting oxide heterostructures exhibit unique properties beyond those of individual components, but their design requires an understanding of energetic and kinetic controls at MOFs–substrate interfaces. Although structure relationship has been widely applied in heterostructure design, it overlooks the interplay between organic ligands, or linkers, and substrate, which defines the kinetics and energetics in the final structure. Herein, we used zeolitic imidazolate frameworks (ZIF-8) on ZnO as a model system to evaluate this interplay via in situ monitoring and simulations.
The Impact
This is the first piece of systematic research on unraveling the physical controls of linkers on metal oxide surface step kinetics by in situ atomic force microscopy monitoring combined with ab initio molecular dynamics (AIMD)—a realistic simulation of complex systems—and density-function theory (DFT), which control the dissolution kinetics of substrate, and therefore the overgrowth kinetics of MOFs/metal oxide heterostructural materials.
Summary
This research addresses two topics that are not well understood in literature: the interplay between organic linkers and substrates during MOF crystallization, as well as the mechanisms that control heterostructure formation in solutions. It is the first piece of systematic research on unraveling the physical controls of organic linkers on metal oxide step kinetics, which controls the overgrowth rate of overlayer MOF materials by in situ monitoring combined with ab initio molecular dynamics and DFT theory. The fact that the linker 2-methyl-imidazole (2-MIM) can simultaneously act by three different mechanisms is fascinating (“dissolution-promoter,” “step-pinner,” and “terrace-binder”), and is also a unique concept given that all three are observed on a single surface—the ZnO (001) face. In contrast, only “dissolution-promoter” and “terrace-binder” are identified for the ZnO (100) face—this is a novel observation. These differences define the ZnO-face-specific growth kinetics of MOF/ZnO heterostructures. In principle, our mechanism should have a wide implication for other biological, energy, and environmental heterostructural materials formation, especially in solution conditions.
PNNL Contact
Jinhui Tao
Materials Scientist, Pacific Northwest National Laboratory
Jinhui.tao@pnnl.gov
James De Yoreo
Battelle Fellow, Pacific Northwest National Laboratory
James.DeYoreo@pnnl.gov
Jun Liu
Battelle Fellow, Pacific Northwest National Laboratory
Jun.Liu@pnnl.gov
Funding
This research was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Division of Materials Sciences and Engineering, Synthesis and Processing Sciences Program. XRD and SEM characterization were performed in the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy's Office of Science Biological and Environmental Research program located at Pacific Northwest National Laboratory (PNNL). Computational resources were provided by PNNL’s Research Computing and EMSL user facility.
Published: August 7, 2020
J. Tao et al, “Controlling Metal-Organic Framework/ZnO heterostructure kinetics through selective ligand binding to ZnO surface steps.” Chemistry of Materials, (2020) http://doi.org/10.1021/acs.chemmater.0c02123