Single-Crystal to Single-Crystal Insertions of Carbon Dioxide Into Bridging Copper Hydride Bonds
Studying single crystal reactions provides molecular-level insight into catalytic processes
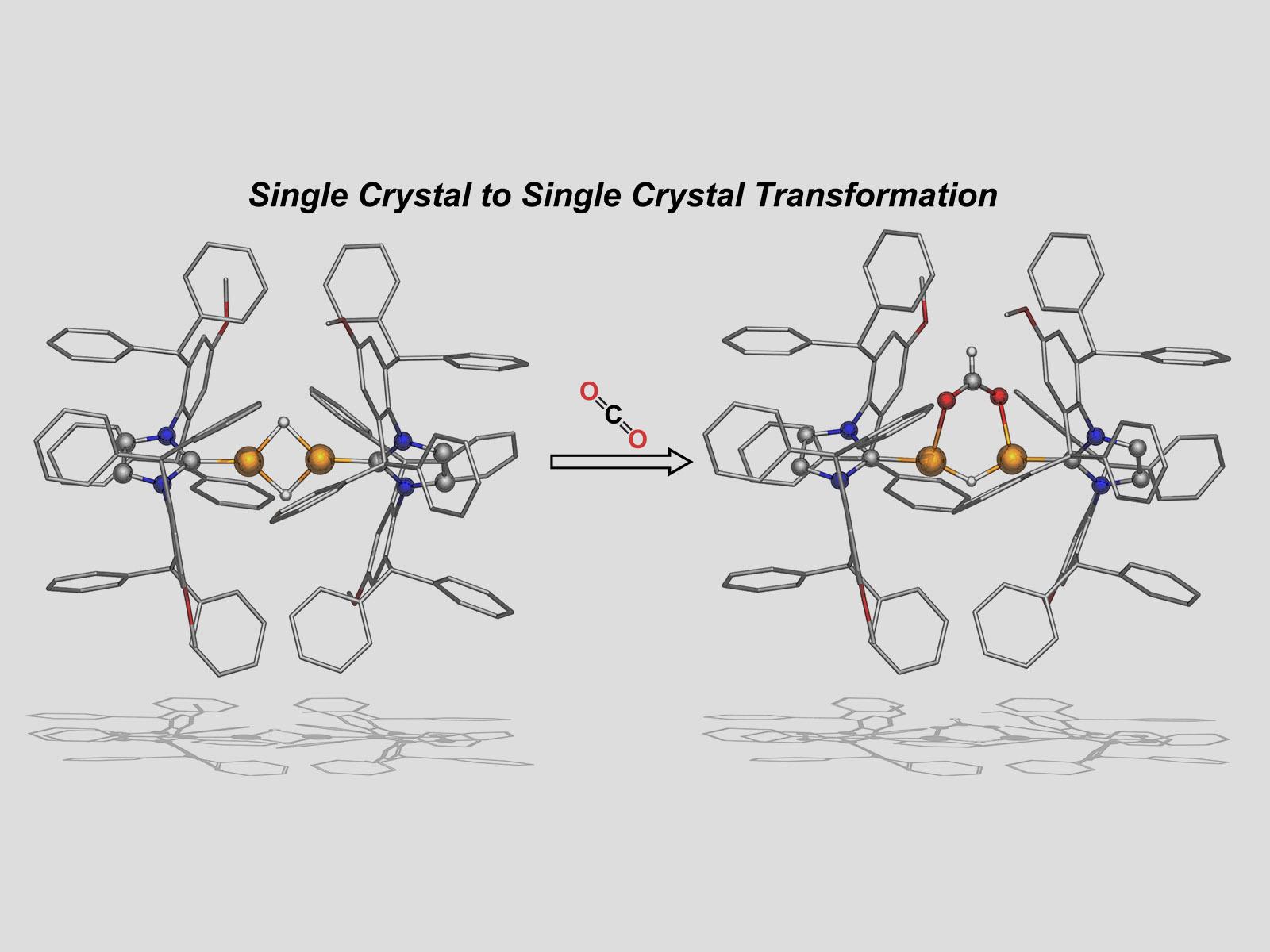
Carbon dioxide inserts into copper–hydrogen–copper bonds in a solid crystal.
(Image by Ba Tran | Pacific Northwest National Laboratory)
The Science
Many industrially relevant catalytic reactions are run at large scales with solid catalysts. While these processes may be efficient, identifying precise reaction mechanisms can be challenging. Researchers used a gas–solid reaction between crystals of a copper (Cu)-containing molecule and carbon dioxide (CO2) to study CO2 insertion into Cu-H-Cu bonds. They observed two SC-SC insertions of CO2 into the Cu-H-Cu bond, which demonstrated unexpectedly high reactivity. When the solid crystals were dissolved in a solvent, the complexes cleanly break apart. This work identified a new route to copper-based formate complexes that may be relevant in CO2 catalysis.
The Impact
Converting CO2 to fuels is an important part of effectively closing the carbon cycle. In this challenging process, understanding the different potential mechanisms enables researchers to create optimized catalysts. This work uses molecular complexes to study potential mechanisms at play in solid heterogeneous catalysts.
Summary
Insertion of CO2 into metal hydride bonds is a pivotal step in the catalytic reduction of CO2 to fuels. Studies of molecular complexes can provide a useful and highly detailed understanding of reaction mechanisms. This work studied the reaction of CO2 and a solid copper (Cu)-based organometallic complex in a single-crystal to single-crystal (SC-SC) transformation, providing a crucial link between homogeneous and heterogeneous catalysis. When exposed to CO2 gas for 15 minutes, insertion of CO2 into a bridging Cu-H-Cu bond converts SCs of [(IPr*OMe)CuH]2 to SCs of a dicopper formate hydride [(IPr*OMe)Cu]2(1,3-O2CH)(H). Remarkably, after three hours of exposure to CO2, a second SC-SC reaction occurs through insertion into the remaining Cu-H-Cu bond. The bridging formate complexes provide insights into proposed intermediates in reduction of CO2. Upon dissolution in a solvent, both of the bridging formate crystals rupture cleanly into mononuclear copper complexes. The gas-solid reactions provide insight into a new reaction pathway for these complexes. In general, insertion of any substrate into a bridging hydride is disfavored compared to terminal metal hydride bonds, so these results illustrate surprisingly high reactivity of the bridging hydride bonds.
PNNL Contact
Ba Tran, Pacific Northwest National Laboratory, ba.tran@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division, Catalysis Science Program, FWP 47319.
Published: September 8, 2023
Patrick E., M. Bowden, J. Erickson, R. M. Bullock, and B. Tran. 2023. Angew. Chem. Int. Ed. [DOI: 10.1002/anie.202304648]