Separating Critical Materials from Dissolved Batteries
A straightforward method can separate critical metal ions from an unconventional source

A gel-based system allows researchers to separate metal ions from a mixture that models battery composition.
(Illustration by Mike Perkins | Pacific Northwest National Laboratory)
What happens when a battery dies? Current rechargeable batteries contain a mixture of metals, some of which are becoming rarer by the day. Keeping these critical materials available for use requires finding new and innovative ways to recycle modern energy technologies, such as batteries.
However, the more complicated the device, the harder it is to effectively extract individual elements. To meet this challenge, researchers are developing new strategies to separate one element from another. Many current separation techniques require substantial energy inputs or highly specialized and expensive components.
Researchers at Pacific Northwest National Laboratory (PNNL) demonstrated a simple, new, and effective way to separate metal ions from a simulated battery electrode mixture. Their process relies on fundamental chemical principles and requires no specialty chemicals, binding agents, membranes, or toxic solvents. Instead, it uses a common gel loaded with a precipitating agent.
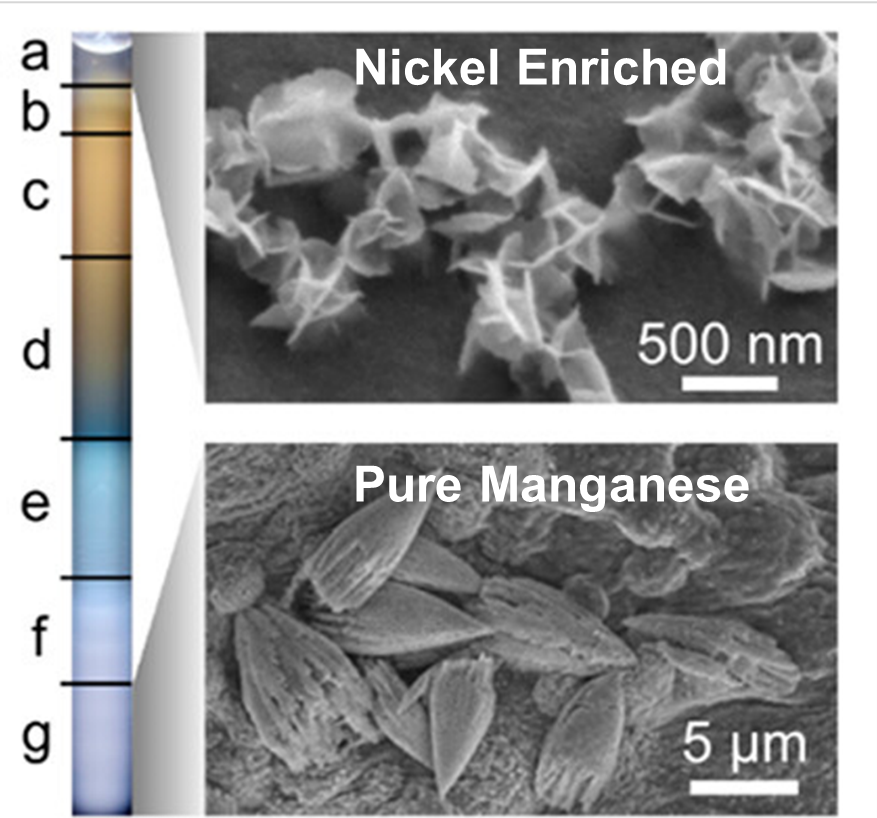
The process is highly tunable. The base gel structure is straightforward to prepare, and the precipitating agent, in this case, an incredibly common salt molecule, can be changed at the time of synthesis. Depending on the metals in a target separation, the team can use a different precipitating agent customized to the specific application.
“The beauty in this process is its simplicity,” said PNNL Materials Scientist and corresponding author Elias Nakouzi. “Rather than relying on high-cost or specialty materials, we pared things back to thinking about the basics of ion behavior. And that’s where we found inspiration.”
The separation works because different ions travel through the gel and form solids at different rates. As a mixed solution moves through a gel-filled tube, the metal ions precipitate out at different times. The metal that forms solids fastest reacts first and uses up the precipitating agent. The process then continues gradually, with the next fastest solid forming until all the ions have separated.
The current work, the subject of a provisional patent, focused on a proof-of-concept demonstration in batches at small scales. Solutions containing mixtures of lithium, manganese, cobalt, and nickel, all important in battery materials, were placed on top of the gel. The liquid was then allowed to move through the gel, leading to ion separation.
Compared to just mixing the precipitating agent and the ions in a solution without the gel, this approach represents a substantial improvement. Simple mixing produces solids that are a nearly equal combination of the different metals. The gel-based separation, meanwhile, results in a maximum purity of 96 percent for the final ion. Future work will focus on scaling this process up to larger capacities and identifying efficient ways to recover the separated salts.
The simplicity of the concept makes it powerful. While this work focused on separating ions from a model battery system, the approach is applicable to other mixtures of metals. Future work will study different unconventional sources of critical materials.
“We’re still in very early stages of this work,” said PNNL Materials Scientist Qingpu Wang. “I think we’ve only scratched the surface of the massive potential for separating many different ions. We already have promising results on separating rare earth metals from dissolved magnets. We plan to take this approach and apply it to more systems in PNNL’s new Non-Equilibrium Transport Driven Separations Initiative.”
Published: January 24, 2024