Revealing the Transient Intermediate Responsible for Impurity Incorporation
Simulations show an amorphous intermediate that allows impurities to incorporate into crystallizing calcium carbonate
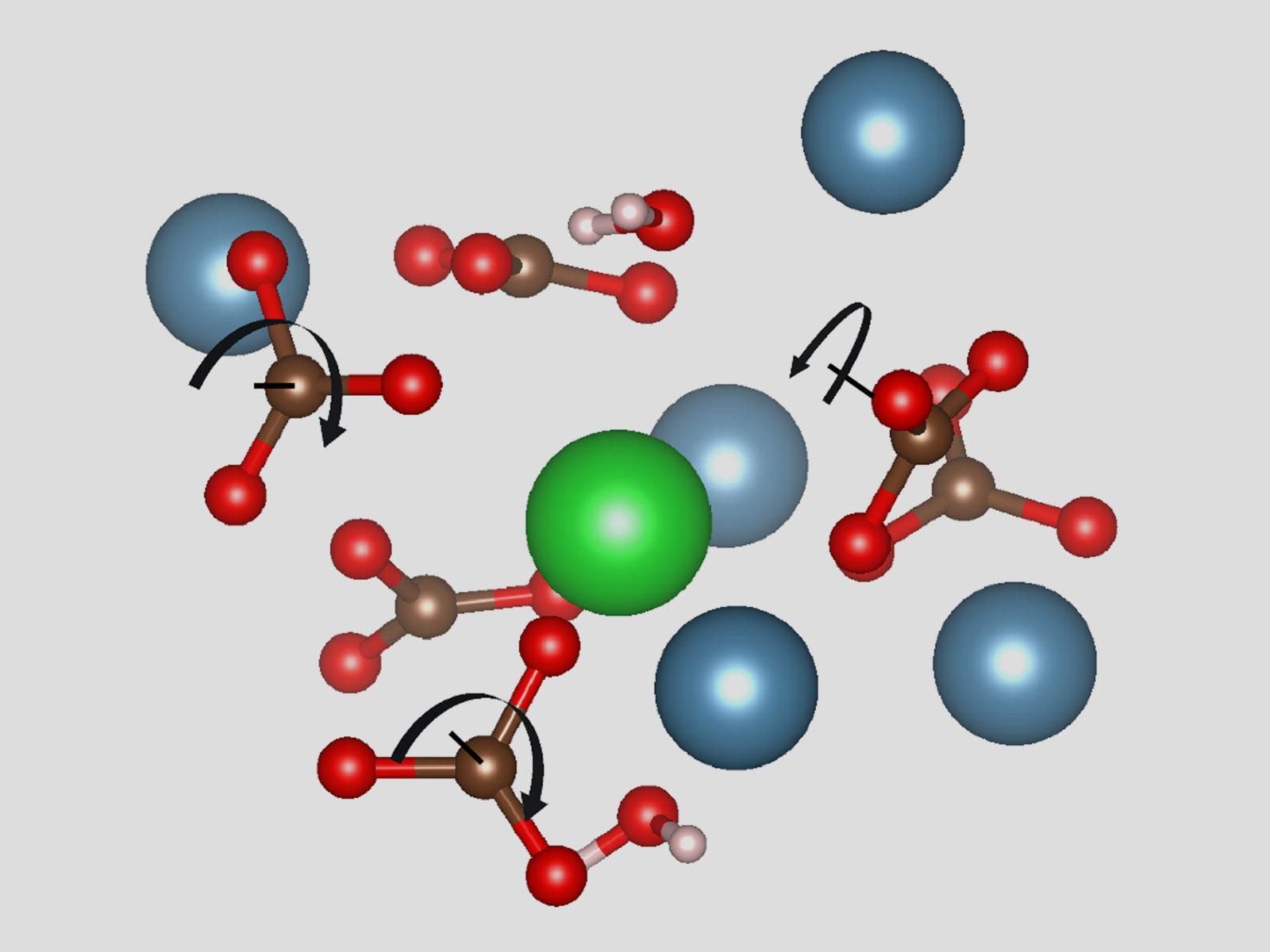
Amorphous carbonate precursors can modify their structure, facilitating the incorporation of impurities.
(Image by Sebastien Kerisit | Pacific Northwest National Laboratory)
The Science
Carbonate minerals are common in geological and biological systems. They form through amorphous intermediates, which can incorporate impurities that have a significant effect on the final crystalline carbonate structure. New research used simulations to study barium (Ba) incorporation into amorphous calcium carbonate (ACC). Researchers found that the rotation of the carbonate ions and overall local density changes in ACC allowed Ba to incorporate into the material. The simulations highlight the importance of the flexible structure of ACC to the inclusion of impurities and how the structural changes ripple throughout the material.
The Impact
Mitigating the effects of carbon dioxide requires a diverse range of solutions, including mineralization-based carbon storage. Understanding how carbonates form at a molecular level is essential for designing effective mineralization systems. This work identifies how ACC incorporates impurities with atom level detail. These results and their corresponding simulations will help researchers develop more accurate models of carbonate crystallization from amorphous starting points. While this research focused specifically on Ba and ACC, future studies will broaden the scope of the impurities and carbonate materials.
Summary
Carbonate minerals are important in many geological and biological processes. Carbonate minerals often first form as a short-lived amorphous phase that converts into a persistent crystalline phase. While impurities incorporate in carbonate minerals and significantly affect this process, the root cause of their effect is poorly understood. Researchers used ab initio molecular dynamics simulations to model how ACC changes and incorporates impurities, specifically focusing on Ba. The results show how the ACC intermediate can modify its atomic structure to incorporate Ba. They clearly demonstrate structural changes caused by an impurity ripple far into the amorphous phase, explaining why even low concentrations of impurities have been found to drastically affect carbonate crystallization. The structural predictions were validated by comparison with experimental data. This work will help enable the development of predictive models of impurity incorporation in carbonate minerals and carbonate crystallization in general.
PNNL Contact
Kevin Rosso, Pacific Northwest National Laboratory, kevin.rosso@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences program (FWP 56674) at Pacific Northwest National Laboratory (PNNL). The simulations were performed using PNNL Research Computing and EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at PNNL, which is sponsored by DOE's Biological and Environmental Research program.
Published: September 7, 2023
S.N. Kerisit, M.P. Prange, S.T. Mergelsberg. 2023. Chemical Communications, 59, 6379-6382. [DOI: 10.1039/d3cc00959a]