Revealing Oxygen Mixing Mechanisms at Surfaces
Studying the chemical reactions that occur when oxygen mixes into the surface of oxide materials
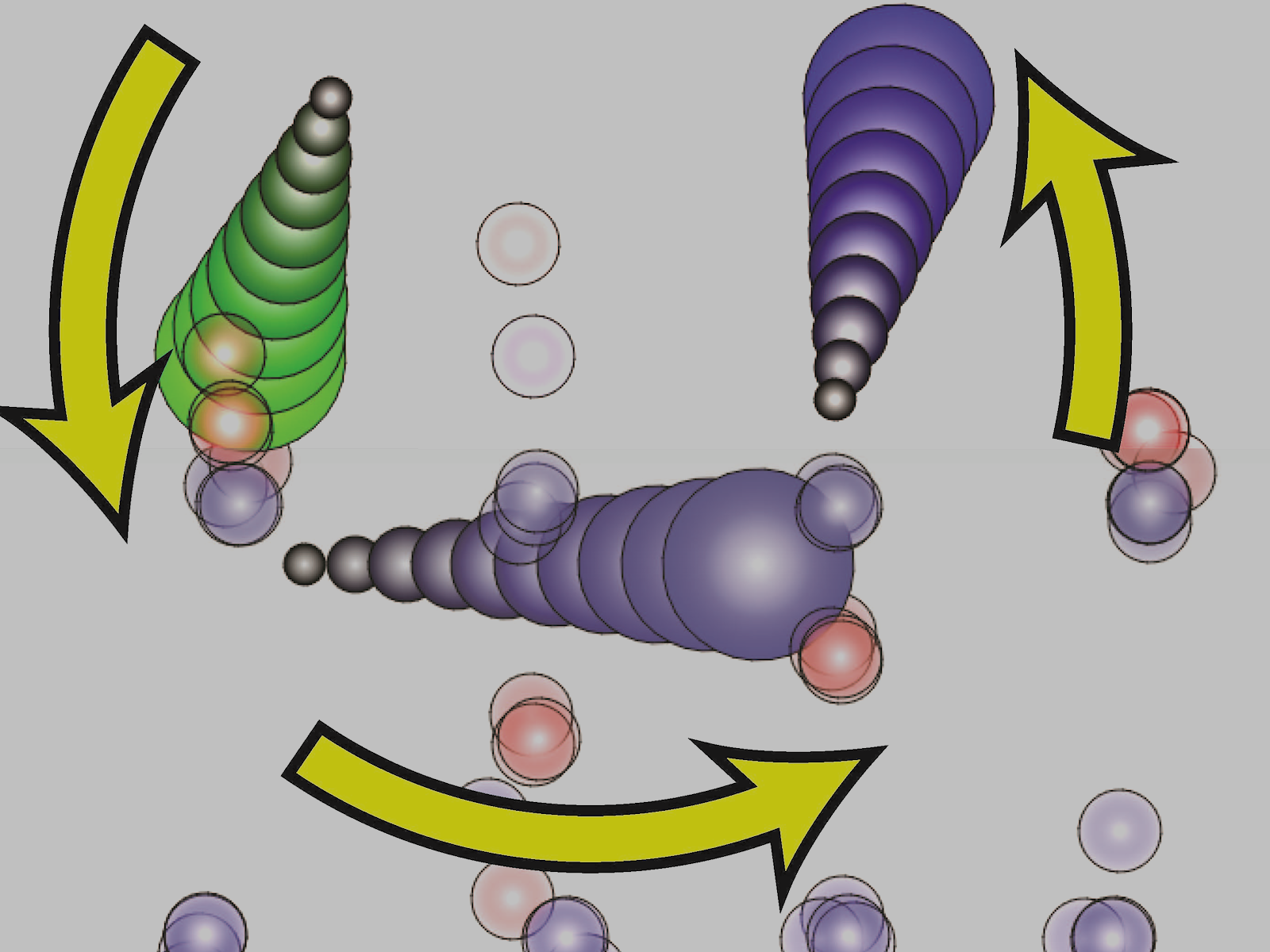
Precisely synthesized oxide systems and advanced simulations allowed researchers to identify an unexpected atomic diffusion mechanism in thin films.
(Image by Pete Hatton and Blas Uberuaga | Los Alamos National Laboratory)
The Science
Depositing thin films of materials is a complex process involving adatom adsorption where individual atoms land on a surface. These adatoms then move around before being fully incorporated into the final material. Researchers used oxide layers with isotopically labeled oxygen to study the movement of atoms into surface layers during thin film deposition. They generated three-dimensional elemental maps and found that oxygen from lower layers moved to the surface of the material. The researchers were able to identify a mechanism where newly added surface atoms “pull up” atoms from the layer below, followed by ring-like rotation that circulates atoms within the material.
The Impact
Thin film deposition from the vapor phase is a key technology that enables commercial fabrication in applications ranging from decorative coatings to computer chips. It also plays an important role in the research and development of new materials and devices for next-generation technologies. Developing a deeper understanding of how thin films form and change can help researchers predict the behavior of new materials. Additionally, many important processes, including degradation and catalysis, occur within the first few layers of materials where these mechanisms might be at play.
Summary
Despite the importance of thin film deposition to the fabrication and development of materials, researchers still lack a comprehensive, predictive understanding of the process. Thin film deposition from the vapor phase is complex, involving adatom adsorption, movement of those adatoms, and incorporation into the growing film. While some simulations can capture important aspects of these processes, the models typically ignore the subsurface diffusion and intermixing processes often observed experimentally. These processes cannot be explained by invoking bulk lattice diffusion at the deposition temperature. Researchers used novel experimental results and advanced computational simulations to reveal that adatoms on an iron oxide film surface act to “pull up” subsurface oxygen and iron. Subsequently, the atoms go through a ring-like rotation mechanism that facilitates mixing. The surface adatom moves into the first layer of the film, kicking out another atom. This second atom moves laterally and kicks out a third atom, which then goes to the surface. The adatom that began on the surface is now in a layer below, while an atom that was originally one layer below is now on the surface. The team developed a simple model incorporating these mechanisms and used it to model the evolution of intermixing as the film grows thicker, which qualitatively agrees with experimental intermixing results. In addition to film deposition, these intermixing mechanisms may occur during other surface-mediated processes such as heterogeneous catalysis and corrosion, broadening the importance of these results beyond thin film deposition.
PNNL Contact
Tiffany Kaspar, Pacific Northwest National Laboratory, tiffany.kaspar@pnnl.gov
Funding
This work was supported as part of FUTURE (Fundamental Understanding of Transport Under Reactor Extremes), an Energy Frontier Research Center funded by the Department of Energy (DOE), Office of Science, Basic Energy Sciences. A portion of the research was performed using EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science user facility at Pacific Northwest National Laboratory.
Published: June 24, 2022
Kaspar, T.C., P.J. Hatton, K.H. Yano, S.D. Taylor, S.R. Spurgeon, B.P. Uberuaga, D.K. Schreiber, (2022) “Adatom-driven oxygen intermixing during the deposition of oxide thin films by molecular beam epitaxy,” Nano Letters, in press. [DOI: 10.1021/acs.nanolett.2c01678].