Revealing Multi-Step Nucleation of a Framework Silicate
A crystalline silicate framework forms through a non-classical, multi-step nucleation mechanism based on structurally precise clusters
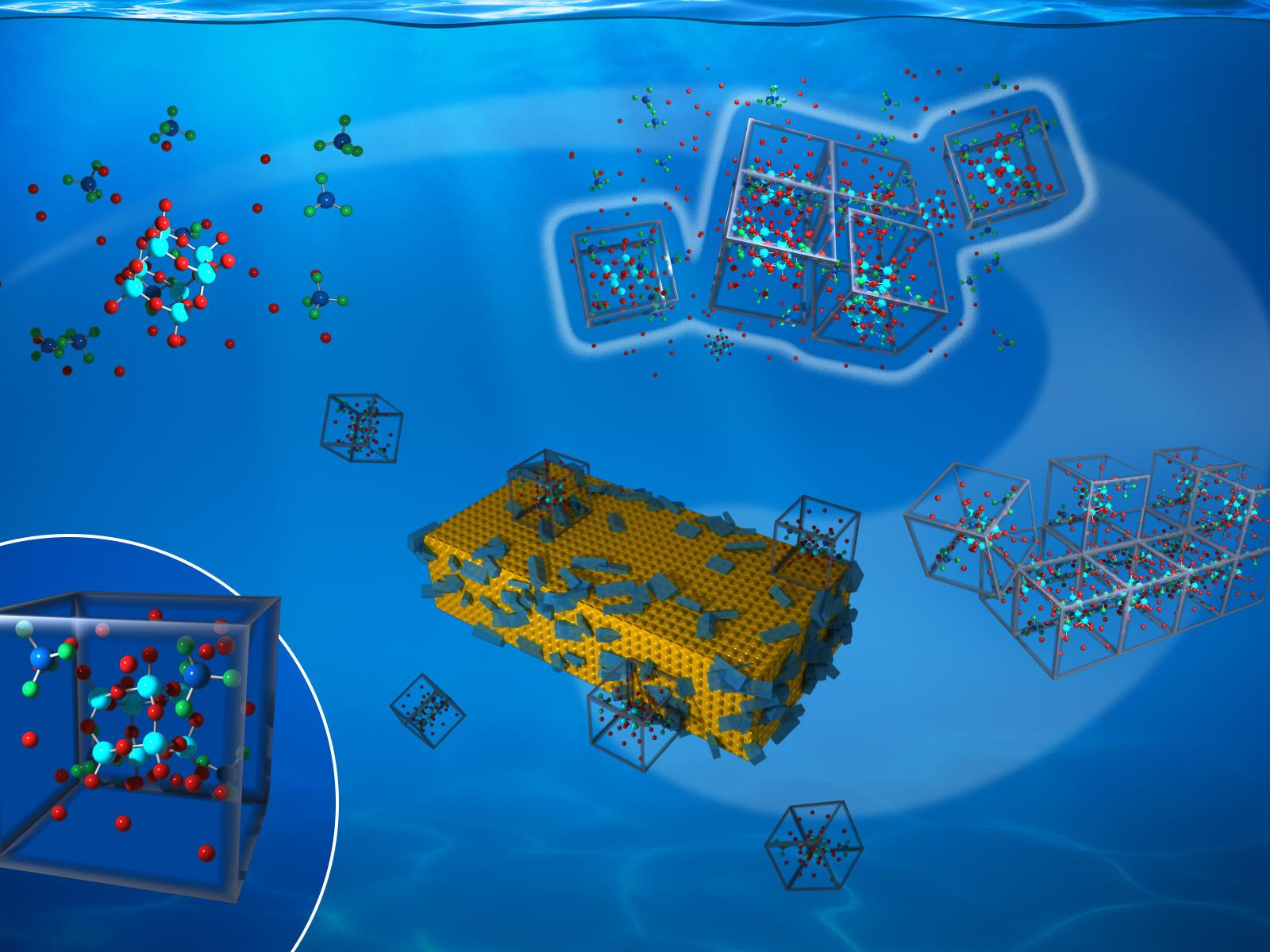
A precisely defined cluster forms a building unit important to the formation mechanism of a crystalline silicate framework.
(Image by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
Materials with open framework lattices are important for a wide range of energy technologies. Understanding how these materials form is necessary for designing precise and controllable synthesis approaches. Researchers studied a crystalline silicate framework and found that it forms by a non-classical, multi-step nucleation mechanism. The mechanism is based on an octameric silicate cage (Q38) that defines a structurally precise prenucleation cluster (PNC) and organizes into a dense liquid precursor with surrounding water and tetramethylammonium ions.
The Impact
Identifying the formation mechanism for open framework lattice materials is challenging. By using in situ measurement techniques, researchers were able to delineate the differences in hierarchical crystallization pathways. The overall results confirm a longstanding hypothesis that the nucleation of porous framework crystals proceeds the assembly of initially formed secondary building units (SBUs). The knowledge produced in this work helps pave the way toward a deep understanding of the synthesis of porous framework materials, with broad implications for the synthesis of functional materials.
Summary
Open framework lattices, such as zeolites, metal-organic frameworks, and clathrates, are critical to numerous energy technologies from catalysis to carbon capture. Thus, understanding how these complex lattices nucleate and grow is an important challenge in energy sciences. In the case of open framework lattices, pre-organized multi-ion SBUs have long been proposed as fundamental building blocks of the forming crystals. However, detailing the progress of multi-step reaction mechanisms in going from monomeric species to stable crystals and defining the structures of the possible intermediate SBUs remained an unmet goal. Combining in situ nuclear magnetic resonance, small-angle X-ray scattering, and atomic force microscopy, researchers show that crystallization of a framework silicate, cyclosilicate hydrate, occurs through assembly of a structurally precise PNC. The PNC includes a cubic octameric silicate polyanion formed through cross-linking and polymerization of smaller silicate monomers and other oligomers. The PNCs are stabilized by hydrogen bonds with surrounding water and tetramethylammonium ions (TMA+), which form a dense liquid network of [(TMA)x(Q38)·nH2O](x-8) clathrate structures. When the PNC concentration reaches a threshold of about 30 percent of the total silicate species, nucleation occurs within these networks. Further growth proceeds through the incorporation of [(TMA)x(Q38)·nH2O](x-8) clathrate complexes into crystal step edges. Thus, these complexes define the SBU of the final crystal. These findings provide a clear picture of the multi-step nucleation process by which SBUs build a framework silicate lattice with implications for the synthesis of both functional materials and natural minerals.
PNNL Contact
Jim De Yoreo, Pacific Northwest National Laboratory, james.deyoreo@pnnl.gov
Funding
This research was performed at Pacific Northwest National Laboratory with support from the Department of Energy (DOE), Office of Science (SC), Basic Energy Sciences program (BES), Division of Materials Sciences and Engineering, Synthesis and Processing Science program, PNNL FWP 67554. Measurements were performed at EMSL, the Environmental Molecular Sciences Laboratory, a DOE SC user facility at Pacific Northwest National Laboratory. X-ray scattering was performed at the University of Washington (UW) with support from BES, as part of the Energy Frontier Research Centers program through CSSAS─The Center for the Science of Synthesis Across Scales. This work also benefited from the use of the SasView application. SasView also contains code developed with funding from the European Union’s Horizon 2020 research and innovation program under the SINE2020 project. The authors acknowledge the use of facilities and instrumentation supported by the National Science Foundation through the Major Research Instrumentation program and the UW Molecular Engineering Materials Center, a Materials Research Science and Engineering Center.
Published: August 8, 2023
Jin, B., Y. Chen, J. Tao, K. J. Lachowski, M. E. Bowden, Z. Zhang, L. D. Pozzo, N. M. Washton, K. T. Mueller, J. J. De Yoreo. 2023. “Multi‐Step Nucleation of a Crystalline Silicate Framework via a Structurally Precise Prenucleation Cluster.” Angew. Chem. Int. Ed. 62, e202303770. [DOI: 10.1002/anie.202303770]