PEEK-ing into the Possibilities of Direct Air Capture
New research reveals enhanced CO2 transport across a polyether ether ketone membrane and water-lean solvent interface
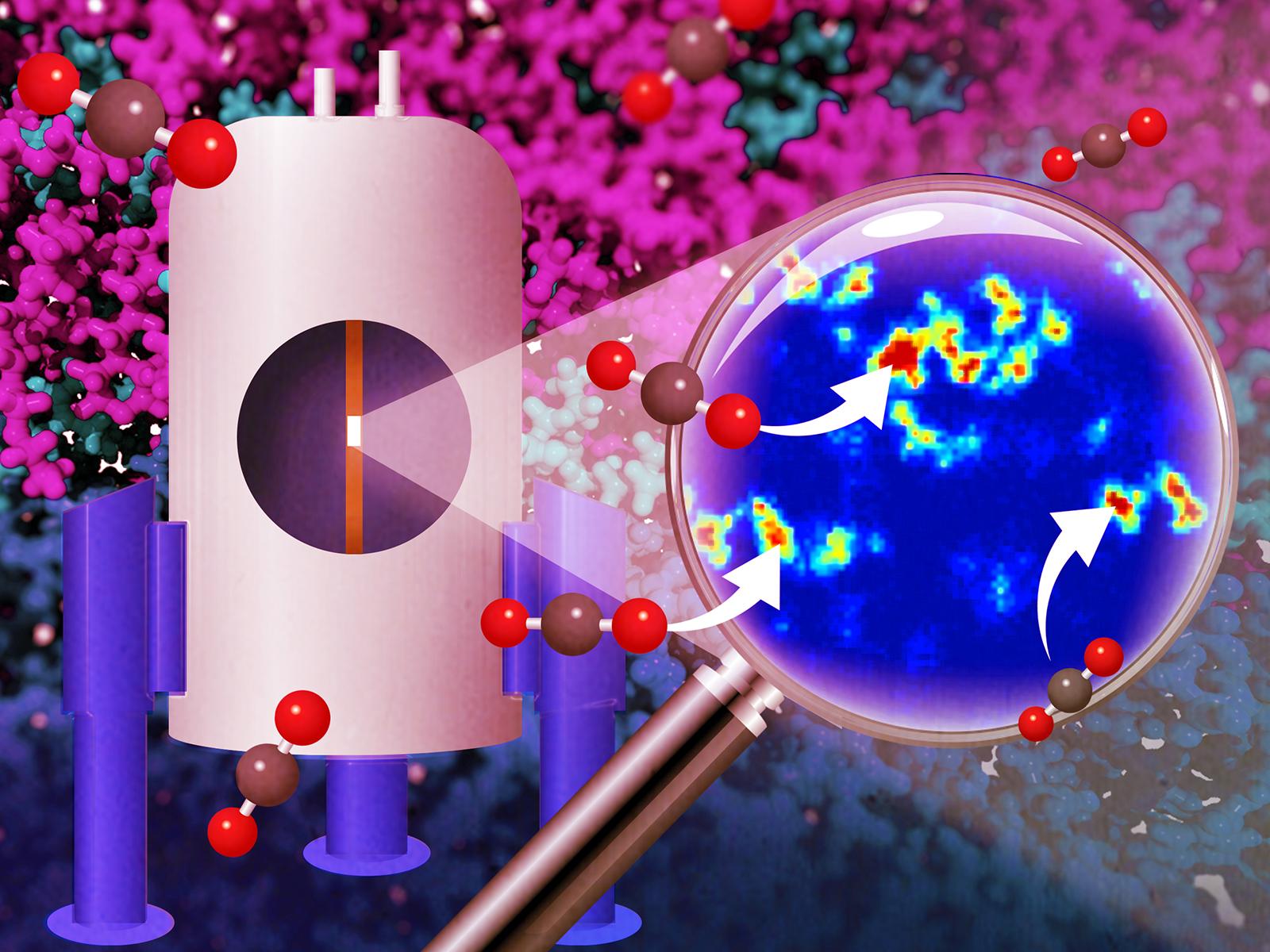
The polyether ether ketone (PEEK)-ionene membrane undergoes a molecular-level reorganization to form channels at the solvent interface that allow for faster CO2 diffusion, as revealed by nuclear magnetic resonance.
(Illustration by Stephanie King | Pacific Northwest National Laboratory)
The Science
Direct air capture (DAC) of carbon dioxide (CO2) is a promising strategy to remove greenhouse gases from the atmosphere. Taking inspiration from living systems, researchers devised a new DAC system featuring a hybrid CO2-selective polyether ether ketone (PEEK)-ionene membrane—analogous to the cell wall—and a water-lean CO2 capture solvent. Then the researchers directly measured the dynamics and speciation between the solvent, membrane, and CO2 under expected DAC operating conditions using advanced nuclear magnetic resonance techniques. When coupled with simulations, their results showed that the membrane undergoes a molecular-level reorganization to form channels at the solvent interface that allow for faster CO2 diffusion.
The Impact
Mitigating greenhouse gas levels to combat climate change remains one of the greatest societal challenges today. DAC is a promising strategy to meet this goal, but current DAC technologies require large amounts of energy to pull CO2 from the air and regenerate the adsorbent. This research shows that enhanced separation performance can be achieved when two separation media are combined to create emergent behavior. The PEEK-ionene material combines the mechanical and chemical robustness of PEEK with the unique solvation-transport properties of ionic liquids. When this material is coupled with a water-lean solvent, the rate of CO2 diffusion across the interface unexpectedly becomes faster than in the bulk solvent. These findings help establish novel design criteria for hybrid membrane-solvent systems.
Summary
Researchers proposed a system that combines a PEEK-ionene membrane with a water-lean N-(2-ethoxyethyl)-3-morpholinopropan-1-amine (EEMPA) solvent coupled to an electrochemical cell for DAC of CO2. They were able to—for the first time—directly measure the dynamics and speciation between the solvent, membrane, and CO2 under conditions expected in DAC operation using advanced nuclear magnetic resonance techniques. Their results show that CO2 diffuses through aliphatic regions in the membrane, not between the imidazolium-bistriflamide ion pairs, with 1H relaxation measurements showing an increase in backbone dynamics. Molecular simulations revealed the addition of EEMPA promotes a molecular-level reorganization in the interface where the addition of imidazoles breaks the membrane’s surface tension resulting in flexible pathways for the migration of CO2 gas molecules in the benzylic regions of the polymer. This restructuring opens channels in the interface between the membrane and the solvent, allowing for faster CO2 diffusion than within the bulk EEMPA solvent and suggesting diffusion across the membrane interface could be tuned to no longer be rate-limiting for CO2 uptake. The approaches we utilized in this study are adaptable to other gas–membrane, membrane–solvent, and gas–solvent separations, allowing for the design of next-generation materials that could perform separations with unprecedented rates and selectivity.
A portion of this work was performed at EMSL—the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility at Pacific Northwest National Laboratory.
Contact
David Heldebrant
Pacific Northwest National Laboratory
david.heldebrant@pnnl.gov
Funding
This work was jointly supported by the Department of Energy, Office of Science, Basic Energy Sciences program, Divisions of Chemical Sciences, Geosciences, and Biosciences and Materials Sciences and Engineering. Computer resources were provided by Research Computing at Pacific Northwest National Laboratory and the National Energy Research Scientific Computing Center, a Department of Energy Office of Science user facility.
Published: September 8, 2023
Walter E., Zhang D., Chen Y., Han K.S., Bazak J.D., Burton S., O’Harra K., Hoyt D.W., Bara J.E., Malhotra D., Allec S.I., Glezakou V.A., Heldebrant D.J.,* Rousseau R.* 2022. “Enhancing Transport of CO2 Across the Interface Between a PEEK Ionene Membrane and a Water-Lean Solvent.” ChemSusChem. doi.org/10.1002/cssc.202300157.