Open Circuit Potential Plays a Critical Role in Controlling the Hydrogenolysis of Benzyl Alcohol in Water
In a water-containing system, the open circuit potential stabilizes positively charged species and lowers the energy barrier of the reaction
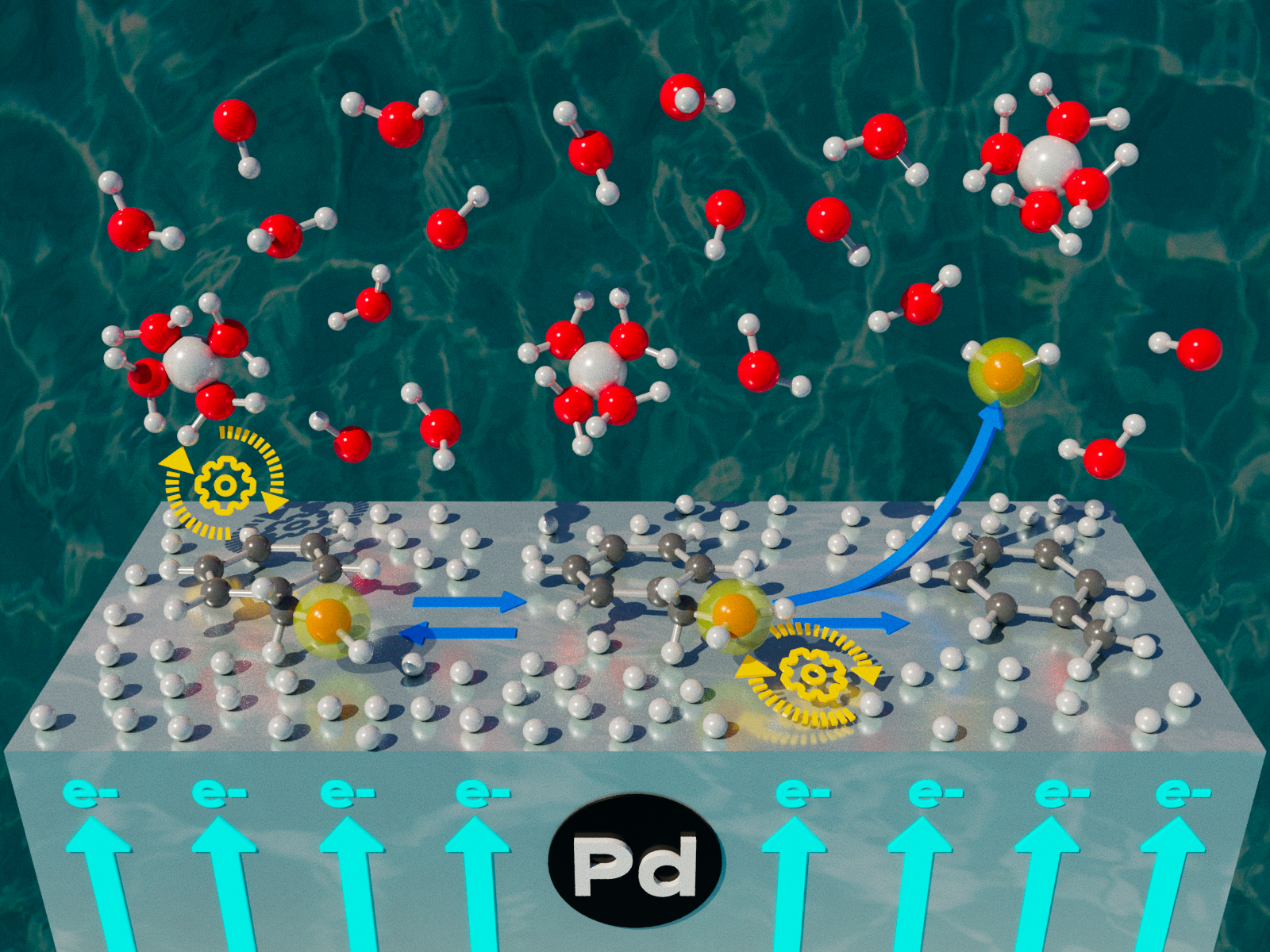
The presence of water, the concentration of hydronium ions, and the hydrogen pressure have important effects on the conversion of benzyl alcohol to toluene in water.
(Illustration by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Many chemical transformations involve adding or removing electrons from molecules. In catalytic reactions, metals commonly play a key role in these conversions. Researchers studied the effects of open circuit potential, the difference in electric potential between the metal catalyst and the environment, on the conversion of benzyl alcohol to toluene in water. Through detailed laboratory and modeling experiments, the team found that the presence of water, the concentration of hydronium ions, and the hydrogen pressure have important effects on the reaction. They also found that the open circuit potential helps stabilize charged states, which decreases the overall activation barriers and enhances the rate of some reactions.
The Impact
Effectively converting organic molecules, molecules with carbon-carbon or carbon-hydrogen bonds, is essential for closing the carbon cycle. Because an open circuit potential is present in all aqueous environments, understanding how it can affect different chemical species is important for finding the right reaction conditions. By identifying the role of open circuit potential in stabilizing charged states, this research deepens scientific understanding of the processes that control driven reactions in water.
Summary
Metals play a key role in many catalytic conversions of organic compounds. Electric potentials that develop spontaneously in the aqueous phase can impose significant effects on the ability of metals to perform the reaction. Identifying how to control these effects remains challenging. To bridge this gap, a team of scientists investigated the impact of pH on the hydrogenolysis of benzyl alcohol to toluene with a carbon-supported palladium catalyst. They performed kinetic experiments and detailed kinetic modeling to quantify the reversibility and kinetic relevance of the different reaction steps. Their results underscore the importance of water, the concentration of hydronium ions, the hydrogen pressure, and the resulting open circuit potential on the reaction. They show that the associated open circuit potential can help stabilize ground and transition states, decreasing the overall activation barriers and enhancing the reaction rates. The next step in this research is broadening its scope and performing a wider set of experiments to assess the generality of these correlations for more hydrogen addition reactions.
PNNL Contact
Karl Mueller, Pacific Northwest National Laboratory
Funding
G.C. is grateful to the Chinese Scholarship Council for the financial support. J.A.L. and O.Y.G. acknowledge the support of the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Division of Chemical Sciences, Geosciences and Biosciences (Impact of catalytically active centers and their environment on rates and thermodynamic states along reaction paths, FWP 47319). Y.L. and W.Z. acknowledge the support of the Open Project Program of Academician and Expert Workstation, Shanghai Curui Low-Carbon Energy Technology Co., Ltd.
Published: February 2, 2023
Cheng, G., Zhang, W., Jentys, A., Gutiérrez, O.Y., Liu, Y., Lercher, A.J. 2022. “Importance of Interface Open Circuit Potential on Aqueous Hydrogenolytic Reduction of Benzyl Alcohol over Pd/C,” Nature Communications, 13, 7967. [DOI: 10.1038/s41467-022-35554-1]