Observing the Mechanism of Protonation Site Switching in Hydrated Nicotine
Proton shuffling in hydrated nicotine at low temperatures occurs via a Grotthuss mechanism, identified through experimental and computational work
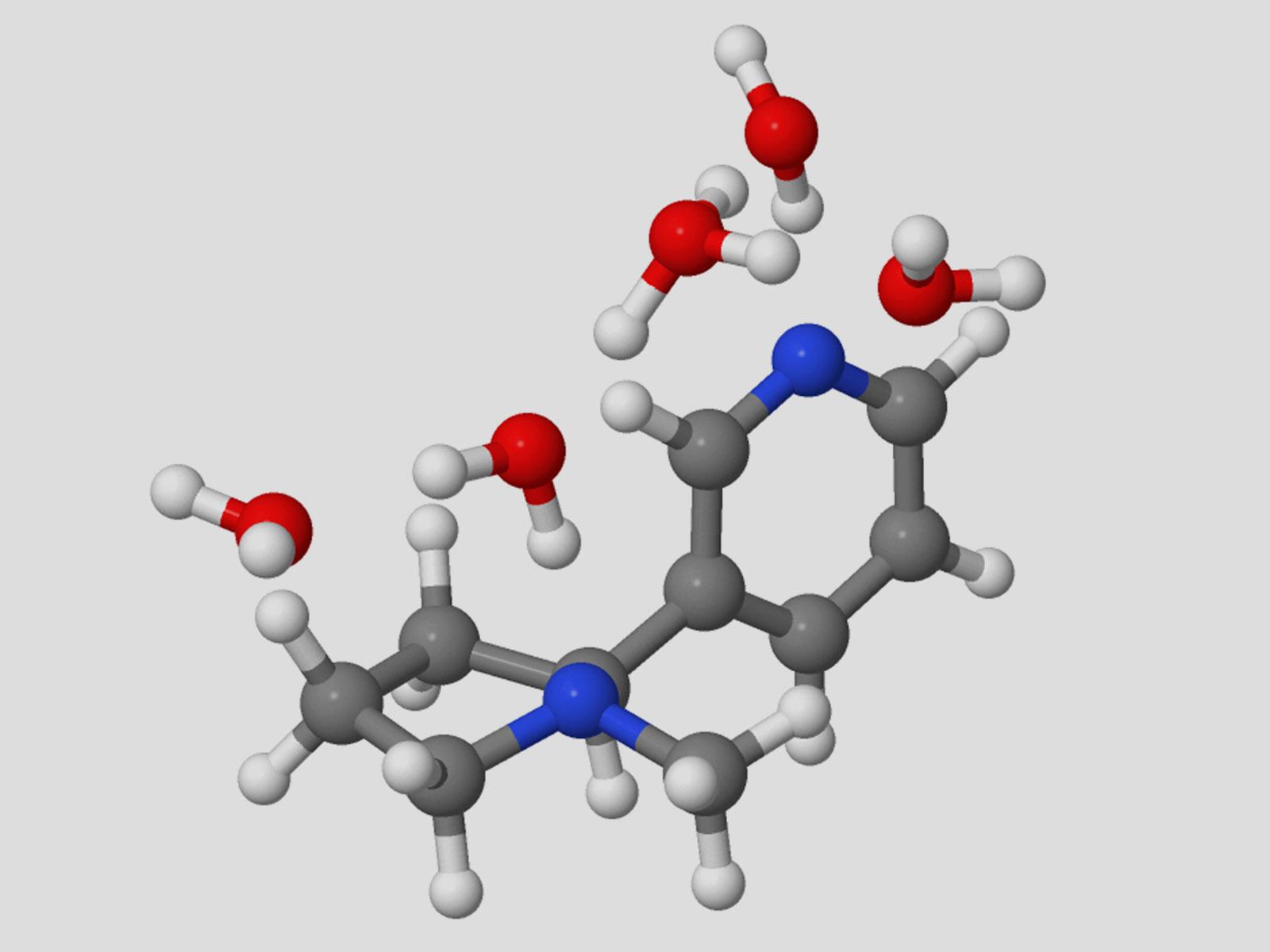
The specific proton transfer mechanism active in hydrated nicotine depends on the reaction temperature.
(Image by Garrett Santis | The University of Washington)
The Science
Proton transfer is central to numerous important systems, from biology to energy storage. Probing the details of proton transfer reactions can provide insight into how to control these processes. Combining experimental and theoretical approaches, researchers examined the behavior of protonated nicotine. They found two different proton transfer mechanisms were active: the Grotthuss mechanism at low temperatures and the bimolecular vehicle mechanism at high temperatures. The bioactive form of nicotine dominates in the presence of as few as five molecules of water. This indicates that this form of nicotine is stable enough to remain available to enter the relevant binding pocket inside a biological system.
The Impact
The biological function of nicotine is directly related to its protonated form, as there are two potential locations for a proton to reside. Understanding the stability and fundamental nature of the active nicotine species can enable researchers to better tune, optimize, and control processes and phenomena involving nicotine. Beyond nicotine, this work provides an example of the power of combining experimental and theoretical approaches in identifying proton transfer mechanisms.
Summary
Nicotine has two potential protonation sites, one of which results in the bioactive, addictive form of the molecule. Researchers probed the proton switching of nicotine in water by infrared spectroscopy and theoretical ab initio calculations. They found that the proton transfer is facilitated via a Grotthuss instead of a bimolecular vehicle mechanism at 130 K, which was unambiguously confirmed with deuterated water experiments. In contrast, the bimolecular vehicle mechanism is preferred at higher temperatures (T = 300 K), as determined by theory. The Grotthuss mechanism for concerted proton transfer results in the production of the bioactive and addictive pyrrolidine protonated nicotine protomer with just five water molecules. Theoretical analysis suggests that proton transfer occurs via hydrogen-bonded bridges consisting of a three-water molecule “core” that connects the pyridine and pyrrolidine protomers. Additional water molecules attach as acceptors to the hydrogen-bonded “core” bridge, lowering the reaction barrier of the concerted proton transfer down to less than 6 kcal/mol, a value that is consistent with experimental observations.
Contact
Sotiris Xantheas, Pacific Northwest National Laboratory and the University of Washington, Sotiris.Xantheas@pnnl.gov
Greg Schenter, Pacific Northwest National Laboratory, greg.schenter@pnnl.gov
Funding
KAKENHI (JP19K22163, JP20K20446, JP20H00372, JP21H04674, and JP21K14585) of JSPS. World Research Hub Initiatives in Tokyo Institute of Technology. The Cooperative Research Program of the “Network Joint Research Center for Materials and Devices” from the Ministry of Education, Culture, Sports, Science and Technology, Japan. Department of Energy, Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, CPIMS Program, under FWP 16249.
Published: September 24, 2024
Okura Y., G. D. Santis, K. Hirata, V. S. Melissas, S.-I. Ishiuchi, M. Fujii, and S. S. Xantheas. 2024. “Switching of Protonation Sites in Hydrated Nicotine via a Grotthuss Mechanism,” J. Am. Chem. Soc. 146, 5, 3023–3030. [DOI: 10.1021/jacs.3c08922]