A Neutral Red-Based System Enables Highly Efficient Direct Air Capture
Researchers designed a new, highly efficient, stable redox-active material from neutral red dye that can efficiently capture CO2 from air.
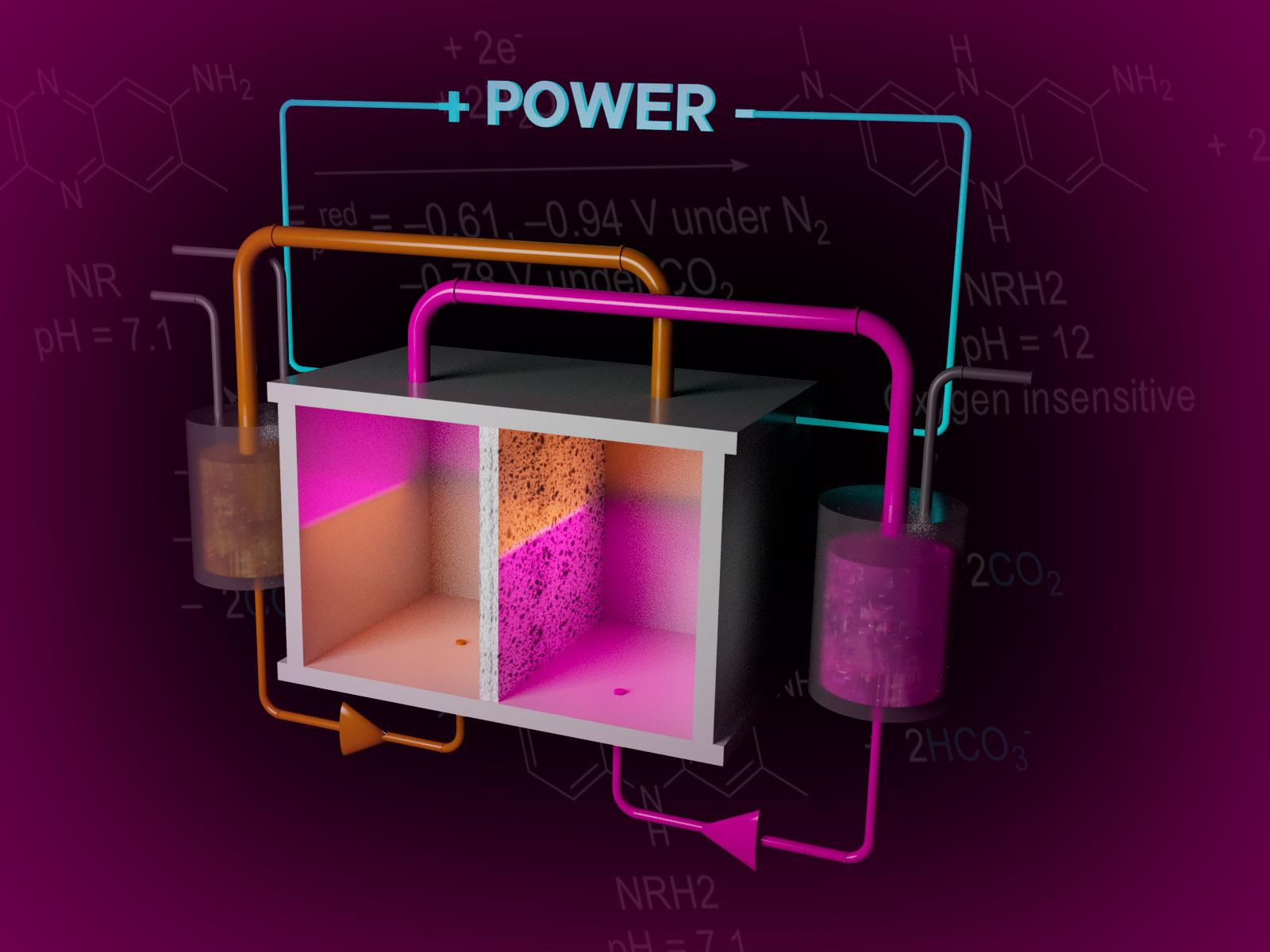
Flow system for CO2 capture from air shows continuous operation for 2 days with minimal loss of capacity due to experimental constraints.
(Illustration by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Direct air capture (DAC) of carbon dioxide (CO2) is a viable option for the mitigation of CO2 emissions and their impact on global climate change. Conventional carbon capture processes from the air require 500 to 800 kilojoule (kJ) of thermal energy per mole (mol) of CO2, which accounts for the majority of the total energy cost of capture. In this work, researchers demonstrate a more energy-efficient electrochemical direct air capture system using neutral red (NR) as a redox-active material.
This DAC system features significant energy savings, with estimated minimum electrochemical energy requirements in the range of 35 kJe/mol using 15 percent CO2 and 65 kJe/mol using air. Though further testing is required for longer-term stability, this system exhibits notable stability over 45 hours in operation with exposure to oxygen. As most electrochemical systems die in oxygen, this represents a big advancement in the field.
The Impact
This work demonstrates the viability of using NR as a new, highly efficient, stable redox-active material that can efficiently capture CO2 from air. This new DAC system features an aqueous solution of low-cost organic molecules that is stable in oxygen, air, and water. These features imply that a carbon capture system based on this redox cycle has the potential for further development and wider applications.
Summary
Researchers designed a new, highly efficient, stable redox-active system based on NR dye that can efficiently capture CO2 from the air. This system involves electrochemical carbon capture by a redox system consisting of NR and its reduced product, leuco-neutral red (NRH2). This system is enabled by the inclusion of nicotinamide as a hydrotropic agent, which increases NR’s solubility in water.
Within this system, NRH2 forms upon application of a suitable electrochemical potential with basification of the aqueous solution to pH 12. Then, the CO2-rich gas stream is introduced to saturate and consequently acidify the solution. Subsequent electrochemical oxidation of the CO2-saturated solution regenerates NR and releases free CO2.
This electrochemical NR-based system demonstrates a high electron utility of 0.39 in a continuous flow cell with an estimated minimum work of 35 kJe/mol from 15 percent CO2. Further exploration using ambient air (410 parts per million [ppm] CO2 in the presence of 20 percent oxygen) as a feed gas shows electron utility of 0.39 in a continuous flow cell to provide an estimated minimum work of 64 kJe/mol of CO2 with the durability of the cells showing >60 percent conservation of electron utilization values after 130 hours. At the lowest current density, this system also demonstrates a high thermodynamic efficiency of 55.7 kJe/mol CO2.
Contact
David Heldebrant
Pacific Northwest National Laboratory
david.heldebrant@pnnl.gov
T. Alan Hatton
Massachusetts Institute of Technology
tahatton@mit.edu
Funding
This work was jointly supported by the Department of Energy, Office of Science, Office of Basic Energy Sciences, Divisions of Chemical Sciences, Geosciences, and Biosciences and Materials Sciences and Engineering.
Published: October 30, 2023
Seo, H., Hatton, T.A., Electrochemical direct air capture of CO2 using neutral red as reversible redox-active material, Nat Commun 14, 313 (2023).
[DOI: 10.1038/s41467-023-35866-w]