Mineral Surfaces Stabilize Organic Matter through Patchy Multilayer Coatings
Molecular simulations highlight the key interactions between mineral surfaces and organic matter that lead to carbon stabilization in soils
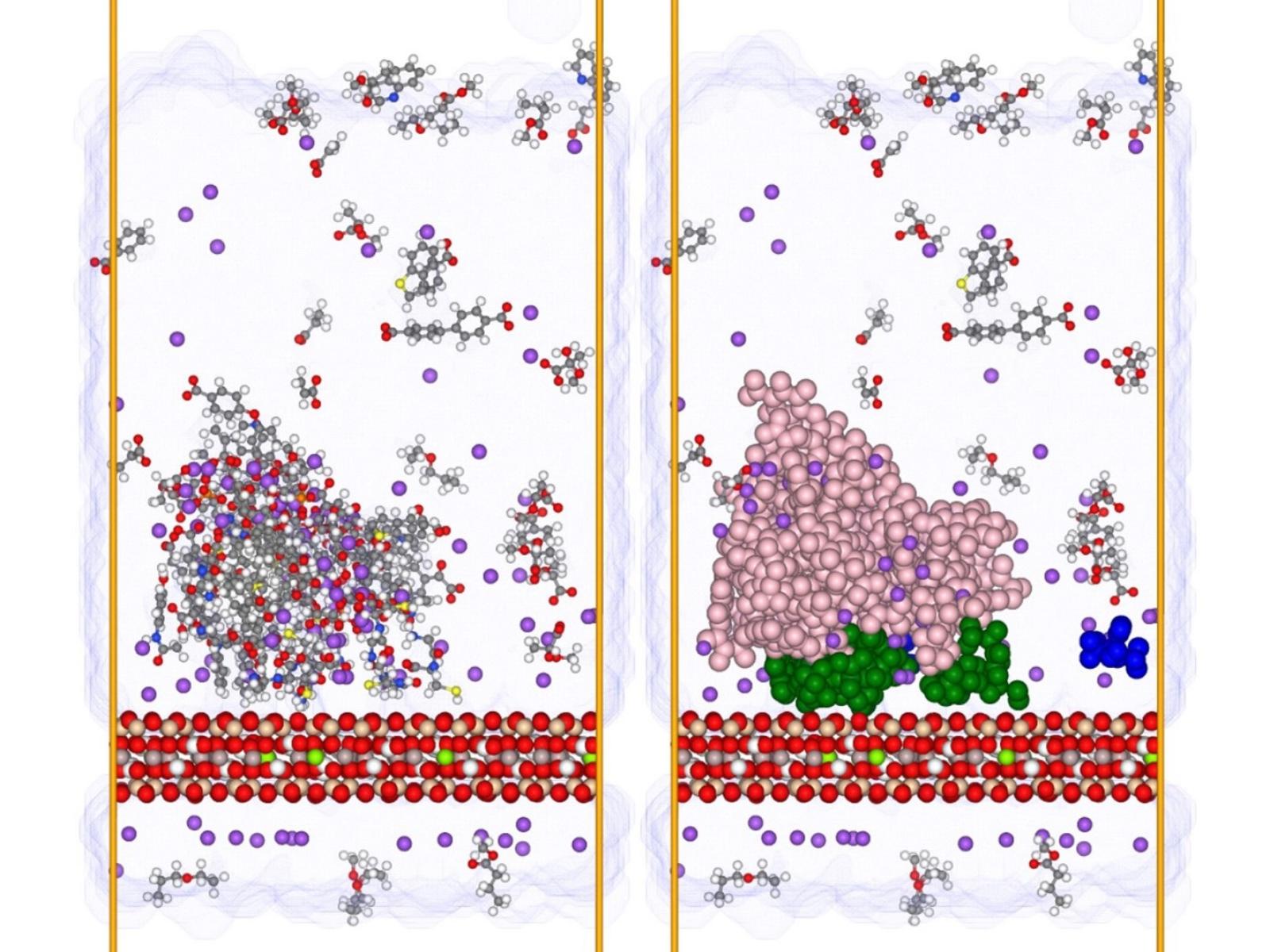
Soil organic matter forms into clusters that attach to mineral surfaces in patchy, multilayered coatings.
(Image by Tom Underwood | Pacific Northwest National Laboratory)
The Science
Soils contain significant amounts of carbon and represent a natural carbon sink that can help buffer carbon dioxide released into the atmosphere. However, the molecular level details of how soil carbon becomes associated with minerals and resistant to degradation remain sparse. New research using molecular simulations has revealed the structure and dynamics of soil carbon associated with representative iron oxide and clay minerals. The team found that the coatings of soil organic matter (SOM) are patchy, dominated by hydrophobic interactions, and are in agreement with an oft-cited hypothetical multilayer model. They also noted that low weight, neutral SOM does not frequently interact with mineral surfaces—an unexpected result based on the prevailing model of SOM behavior.
The Impact
Understanding how SOM behaves is core to developing a picture of how carbon cycles in the environment. This work helps shed light on the mechanisms of soil carbon stabilization. The patchy coatings seen in the simulations have important ramifications for the chemistry of minerals associated with SOM. The patchiness leaves room for water-mediated processes to occur at mineral surfaces close to organic matter, including surface charge accumulation at varying pH as well as dissolution and precipitation. This knowledge can help researchers better identify and develop mineral systems with specific, desired properties.
Summary
The formation of mineral-associated organic matter (MAOM) may explain the slow turnover rates of carbon in SOM. Despite its overall importance, key details about the structure and dynamics of MAOM remain unknown. Researchers employed molecular dynamics simulations to gain insight into the structure of MAOM on the surface of model mineral systems, specifically clay and iron oxide minerals. They used the presence of either sodium or calcium charge balancing counterions to assess the impact of aqueous chemistry on the system. The results are consistent with the hypothesized multilayer sorption (“onion-skin”) model of MAOM and help explain previous observations of a patchy distribution of SOM on mineral surfaces. The SOM coatings are partial and heterogeneous across the surface. This patchiness allows water to retain extensive access to mineral surfaces even when significant SOM sorption occurs. Low molecular weight neutral SOM molecules (less than 200 Da) are observed to infrequently interact with the mineral surfaces and organic matter coatings sorbed on the minerals. The SOM molecules are increasingly labile with decreasing molecular weight. This observation is inconsistent with a central feature of the current predominant soil continuum model of SOM and suggests that further iterations of the conceptual model may be required.
Contact
Kevin Rosso, Pacific Northwest National Laboratory, kevin.rosso@pnnl.gov
Funding
Manuscript Authored by Battelle Memorial Institute Under Contract Number DE-AC05-76RL01830 with the Department of Energy (DOE). The US government retains and the publisher, by accepting this article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan: http://energy.gov/downloads/doe-public-access-plan. This material is based upon work supported by the DOE, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division through its Geosciences program at Pacific Northwest National Laboratory (PNNL) (FWP 56674) and at Princeton University (Award DE-SC0018419). Computational resources were provided by PNNL Institutional Computing. PNNL is a multi-program national laboratory operated for the DOE by Battelle Memorial Institute under Contract No. DE-AC05-76RL01830.
Published: January 10, 2025
Underwood T.R., Bourg I.C., Rosso K. M. 2024. “Mineral-associated organic matter is heterogeneous and structured by hydrophobic, charged, and polar interactions,” PNAS. [DOI: 10.1073/pnas.2413216121]