Ionic Environment Enhances the Activity of Reacting Molecules in Specific Zeolites
Local ionic strength tailors the chemical potential of reacting molecules, leading to high cyclohexanol dehydration activity
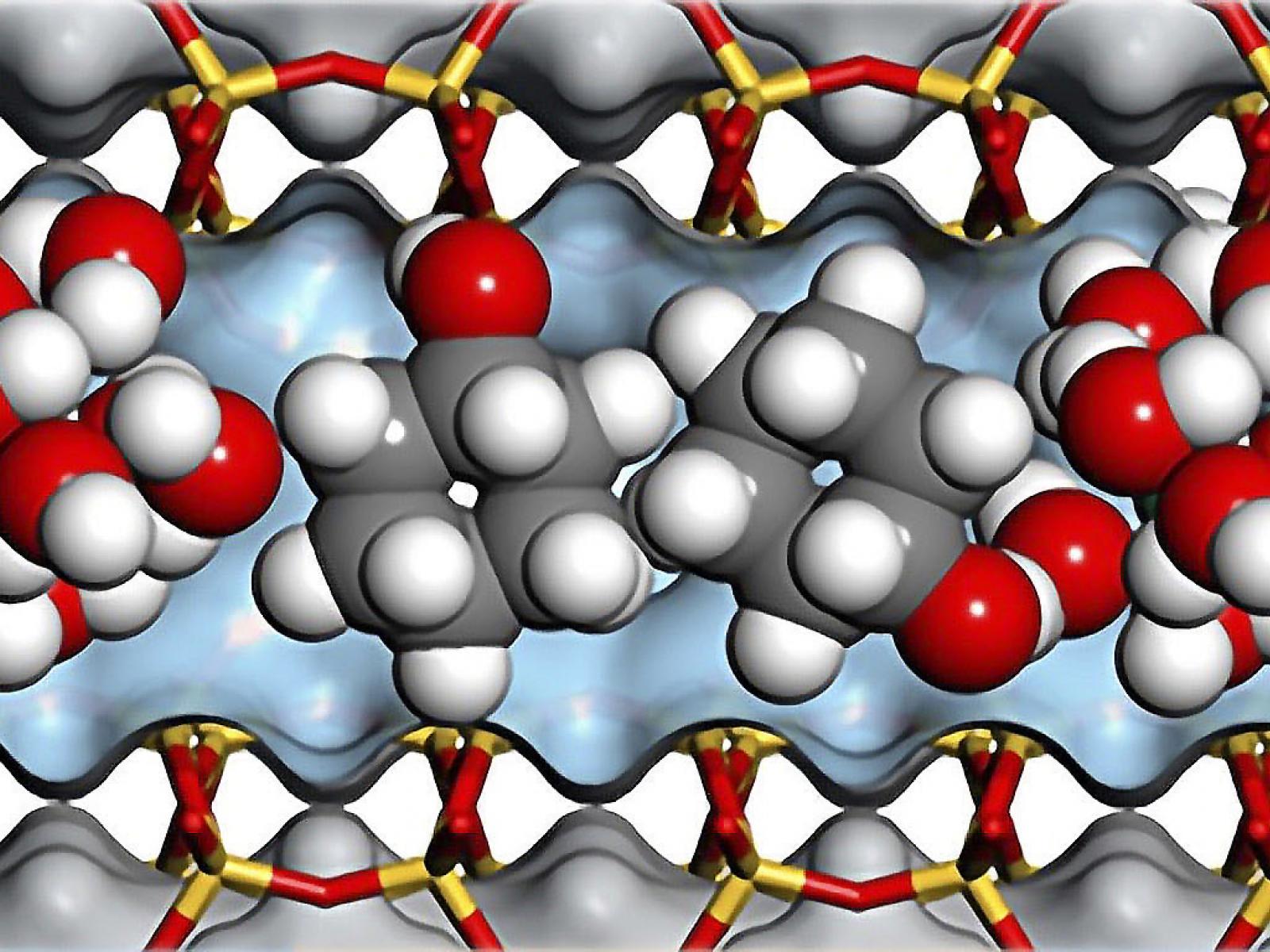
Water molecules within zeolite pores of specified sizes and Brønsted acid site concentrations become hydronium ions that contribute to high cyclohexanol dehydration rates.
(Illustration by Andreas Jentys | Technical University of Munich)
The Science
Zeolites are widely used in the chemical industry for catalysis, but the reason for their high catalytic activity has yet to be fully elucidated. A team of scientists recently resolved some of this lingering uncertainty by demonstrating that the presence of high ionic strength in zeolites enhances aqueous cyclohexanol dehydration reaction rates. Hydrated hydronium ions (H3O+) induce high ionic strength in both H-type mordenite framework inverted (H-MFI) and H-type beta structured (H-BEA) zeolites that have specified pore sizes and Brønsted acid site concentrations. Local ionic strength tailors the chemical potential of the reacting molecules, leading to high cyclohexanol dehydration activity.
The Impact
Bioreactors rely on the chemical conversions that happen inside the tiny pores embedded in zeolites. Some zeolites promote highly efficient biomass energy conversions, but require high temperatures and pressures. This research unveils a potential method for the systematic improvement of catalytic reactivity rates in the aqueous phase, which could be used to enhance energy conversion methods. The next step for this research is to explore increases in reactivity by manipulating the confined space and high ionic strength at even lower temperatures.
Summary
To understand how zeolites work at the molecular level, the researchers created a series of both H-MFI and H-BEA zeolites that have specified pore sizes and investigated the reaction rate of the dehydration of cyclohexanol within these zeolites. They found that in an aqueous environment, hydronium ions within zeolite pores create a microenvironment in the active space that drives the reaction. The density of active sites for the dehydration of cyclohexanol can be optimized by modulating the chemical potential of reacting molecules.
The research team then determined how water molecules arrange themselves inside the pore and how that arrangement changes with the size of the pore. They established that both the bulkiness and the charges of the H3O+ molecules within the zeolite pore affect catalytic activity of the cyclohexanol dehydration reaction by stabilizing the positively charged transition state. This research can be used as a potential method for calculating the optimum relationship between the space available, the presence of hydronium ions, and the reactivity of a catalyst.
Contact
Sungmin Kim
Pacific Northwest National Laboratory
Sungmin.Kim@pnnl.gov
Funding
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences and Biosciences.
Published: July 2, 2021
Pfriem, N., Hintermeier, P. H., Eckstein, S., Kim, S., Liu, Q., Shi, H., Milakovic, L., Liu, Y., Haller, G. L., Baráth, E., Liu, Y., & Lercher, J. A. (2021). Role of the ionic environment in enhancing the activity of reacting molecules in zeolite pores. Science, 372(6545), 952–957.
DOI: 10.1126/science.abh3418