Identifying Propanol Organization and Changes Inside Zeolite Pores
1-propanol converts from a monomer into a trimer at aluminum sites within zeolite pores
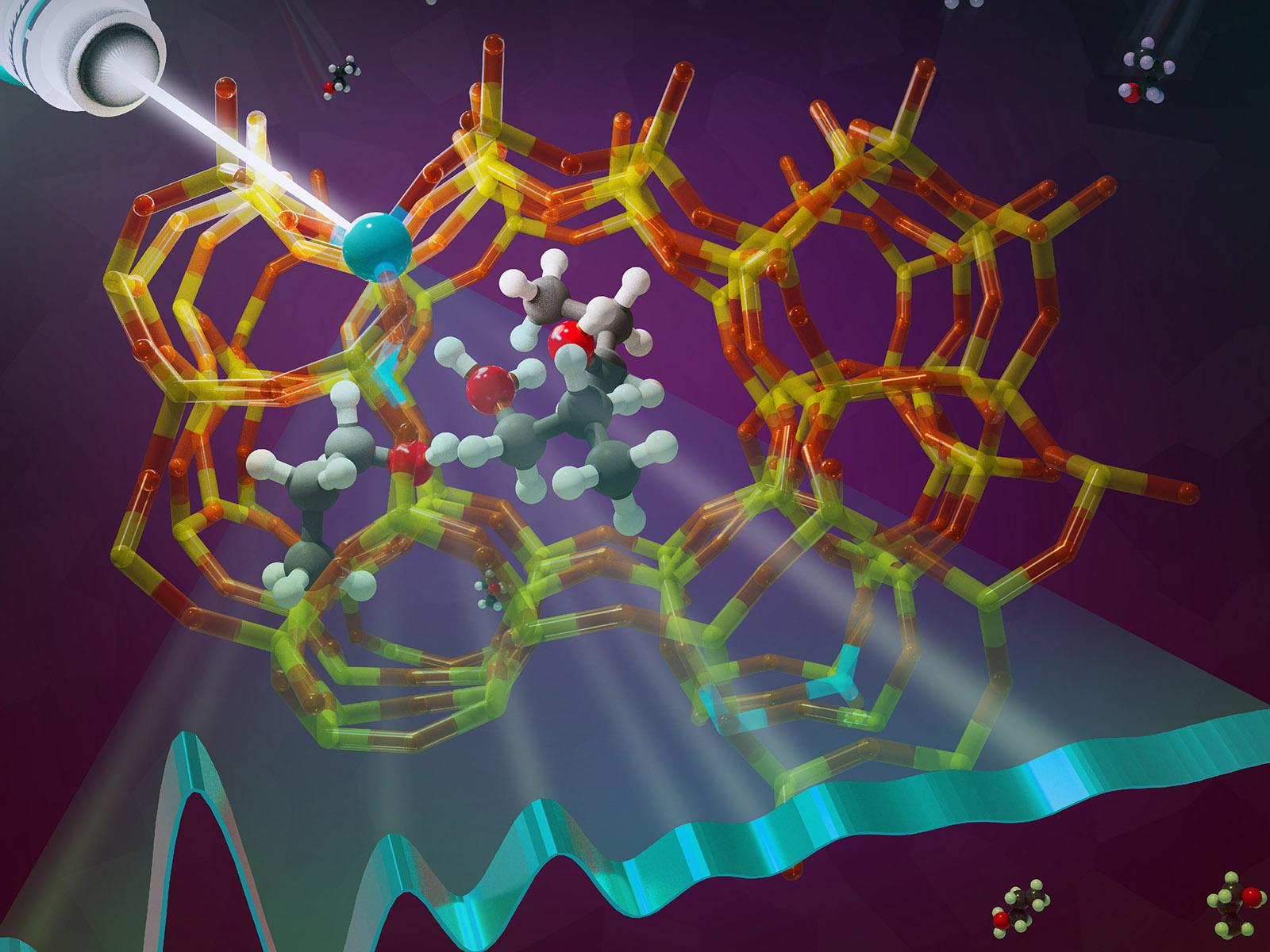
A novel combined experimental and computational approach uses X-ray spectroscopy to identify 1-propanol adsorption in the pores of an aluminum-based zeolite.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
The Science
Zeolites are an important class of materials, commonly used for ion exchange, separations, and catalysis. However, identifying the specific mechanism of catalytic conversions is often challenging. New research combines experimental and simulated X-ray absorption spectroscopy (XAS) of aluminum (Al) to identify the interactions between an alcohol molecule and the zeolite. The spectra show changes in the structure of the aluminum tetrahedra, or T-sites, inside the zeolite pores. The observations allowed researchers to pinpoint the conversion of 1-proponal monomers to trimers as causing the observed changes in the Al T-sites.
The Impact
Understanding how alcohol species evolve in zeolite pores is fundamental to deciphering the elementary steps of their catalytic conversion. In situ Al K-edge XAS combined with XAS calculations using ab initio molecular dynamics (AIMD) trajectories provides a new approach to studying the alcohols inside zeolite pores. The new approach will help researchers better understand interactions between the zeolite catalyst and reactive substrates as well as predict catalyst reactivity.
Summary
Previous work studying 1-propanol adsorption onto the H-MFI zeolite showed that up to three alcohol molecules could adsorb onto a single Al T-site. While the propanol monomers and dimers are well known and characterized species, the presence of the third propanol raised questions about the molecular configurations and structures present. New research used a combination of experimental techniques and simulations focused on X-ray spectroscopies to probe the structure of propanol within the zeolite pore. By identifying changes in the aluminum signal, researchers were able to describe the formation of a propanol trimer as a combined process rather than three individual adsorption events. The results help explain the overall organization of the propanol molecules within the zeolite pore, which can provide a baseline to further probe the local environment in the pore. This approach can also be applied to different zeolites and catalytically relevant systems, providing important information on the interactions between the catalyst and substrate.
PNNL Contact
Mal-Soon Lee, Pacific Northwest National Laboratory, MalSoon.Lee@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, and Geosciences & Biosciences (FWP 47319). PNNL is a multiprogram national laboratory operated for DOE by Battelle under Contract DE-AC05-76RL01830. Computational Resources were provided by user proposals at the Molecular Sciences Computing Facility (MSCF) in the William R. Wiley Environmental Molecular Sciences Laboratory, a Department of Energy (DOE) national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research located at PNNL, and the National Energy Research Scientific Computing Center (NERSC), a U.S. Department of Energy Office of Science User Facility located at Lawrence Berkley National Laboratory.
Published: November 14, 2023
S. Kim, M.-S. Lee, D.M. Camaioni, O.Y. Gutiérrez, V.-A. Glezakou, N. Govind, T. Huthwelker, R. Zhao, R. Rousseau, J.L. Fulton, J.A. Lercher. 2023. “Self-Organization of 1-Propanol at H-ZSM-5 Brønsted Acid Sites,” JACS Au, 3, 2487. [DOI: 10.1021/jacsau.3c00259]