How Proteins Form Tooth Enamel
Protein ribbons are highly active scaffolds for apatite formation
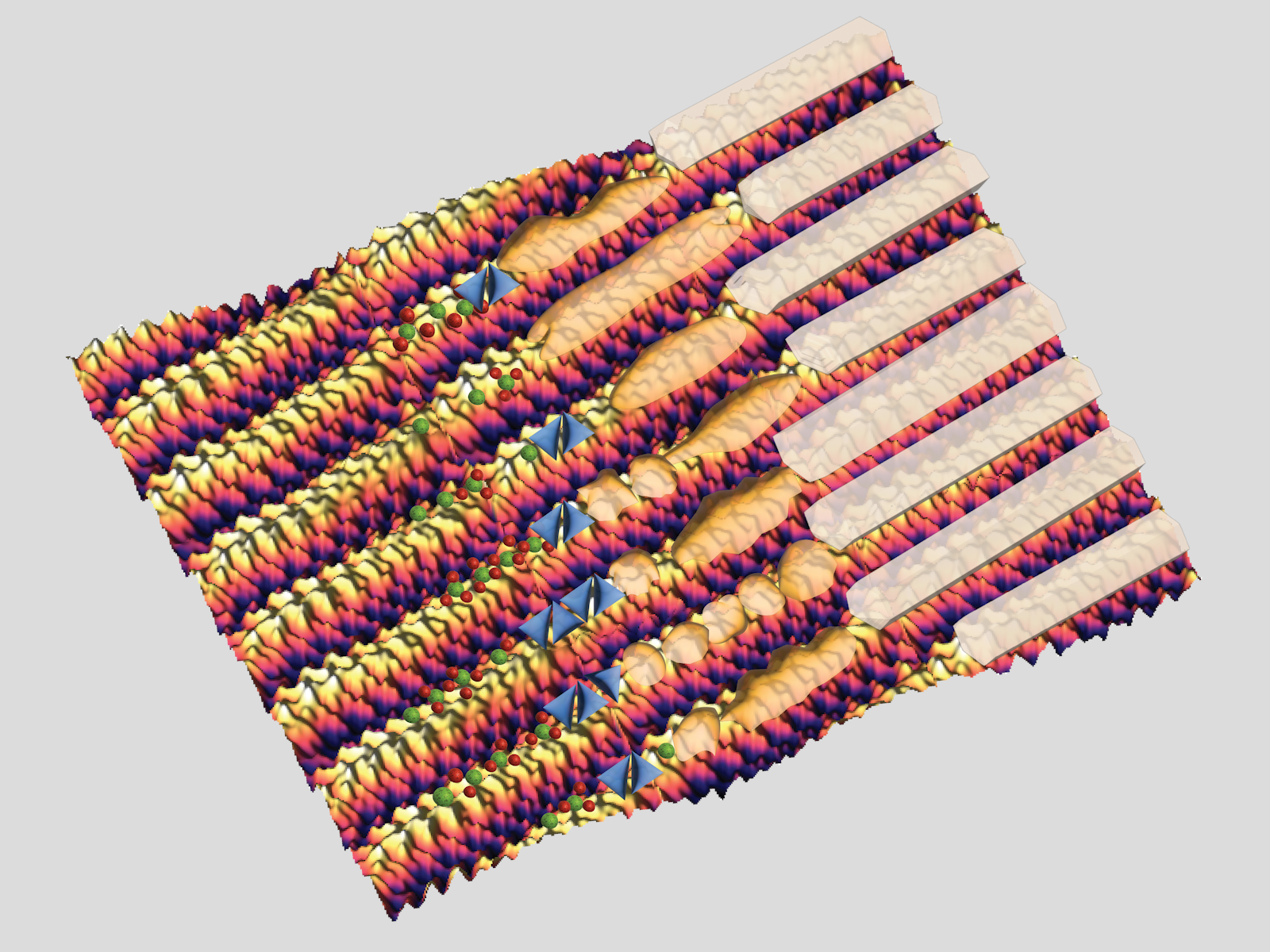
Ribbon-shaped proteins act as scaffolds and template the formation of the hard minerals found in tooth enamel.
(Susrut Akkineni | University of Washington)
Tooth enamel is harder than any other substance in the human body, including bone. Enamel is primarily made of bundles of thin crystals of a calcium phosphate-based mineral known as apatite. However, a protein called amelogenin plays a key role in giving enamel its structure. Amelogenin acts as a scaffold, helping direct the apatite crystals into specific shapes in each bundle.
While the overall process was identified over 40 years ago, scientists still do not understand how enamel forms, especially at the molecular level. Part of the challenge is that the protein scaffold breaks down over time. This makes it difficult to determine how exactly the protein directs the mineral at different stages of enamel development.
New research published in the Proceedings of the National Academy of Sciences USA from an international collaboration between Pacific Northwest National Laboratory (PNNL) and University of Washington scientists, studied how recently identified amelogenin ribbons interacted with an amorphous mineral precursor of apatite. The team used in situ measurements to study the system in solutions that more closely mimic the biological environment than traditional measurements. They found that these ribbons were extremely active at facilitating the formation of the amorphous calcium phosphate, which later turned into apatite crystals.
“Not only were the ribbons extremely active, but they were also quite stable,” said Susrut Akkineni, a University of Washington graduate student currently working at PNNL. “For many years researchers thought sphere-shaped protein aggregates were the scaffolds, so we didn’t expect to see the ribbons work so well.”
Turning a qualitative understanding quantitative
Understanding these processes combined expertise in protein assembly, creating model proteins, running molecular dynamics simulations, and performing in situ measurements. This allowed the team to identify the best conditions for observing tiny calcium phosphate particles, called nuclei, form from ions on the proteins.
The researchers used atomic force microscopy to watch the proteins organize themselves into ribbons. The ribbons then became the mineral scaffold, with the molecular structure verified through high resolution images and simulations. The team tracked how different concentrations of calcium and phosphate ions affected the number of nuclei that formed on the ribbons over time.
The team measured samples from the same time point with a synchrotron-based X-ray diffraction source and a transmission electron microscope. This allowed them to learn more about the protein structure and calcium phosphate mineral phase, respectively. With this information, they developed a mechanistic understanding of the process.
These studies showed that an array of charged sections on the ribbons are primarily responsible for the high mineralization activity. The charged areas give the mineral a helpful template, making it easier for the mineral to form its filament shape along the ribbons.
“The internal structure of the ribbons is quite common across different proteins,” said Jinhui Tao, a PNNL materials scientist. “We can adapt these templating fundamentals to design new mineralization-directing scaffolds.”
The team found that making small changes to the protein sequence, without changing the internal structure, allowed them to alter the overall activity of the system. Two of the protein analogs were the most active scaffolds for templating amorphous calcium phosphate reported to date. This tunability represents an exciting step towards effectively making artificial tooth enamel.
Part of this work was performed at the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility at PNNL. Additional work was performed at the Advanced Light Source at Lawrence Berkeley National Laboratory. Simulations were performed using the Argonne Leadership Computing Facility, an Office of Science user facility. In addition to Akkineni and Tao, the PNNL team included James De Yoreo, a joint appointee with the University of Washington, Miao Song, and Jiajun Chen. PNNL staff collaborated with the team of Cheng Zhu, Samuel Hoff, and Hendrik Heinz at the University of Colorado, Boulder. The also work involved collaborators Stefan Habelitz, the University of California, San Francisco, and Johan Bonde, Lund University.
Published: July 14, 2022