How Does Mg2+ Diffusion Change the Structure and Properties of Fe3O4 Thin Films?
Alternative methods for testing ion movement through materials
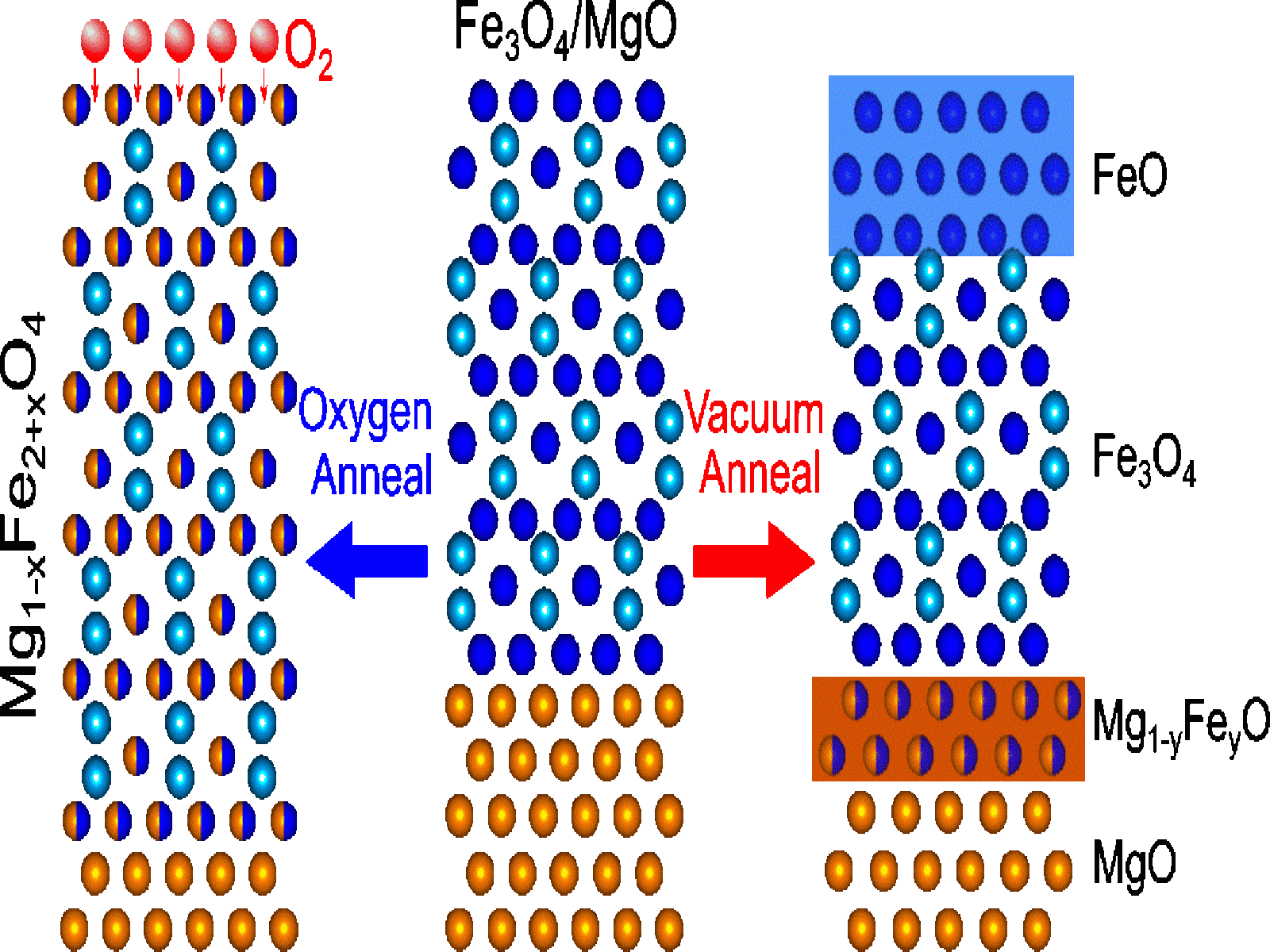
Annealing Fe3O4/MgO films in O2 leads to facile Mg2+ diffusion where the Mg2+ replaces Fe2+ throughout the Fe3O4 later. Annealing the films under vacuum leads to a boundary layer that blocks further Mg2+ diffusion.
(Illustration by Linda Wangoh | Pacific Northwest National Laboratory)
The Science
As society moves towards a new energy future, the need to develop and understand advanced materials for energy storage and conversion continues to increase. Magnesium cation (Mg2+) -based batteries are projected as high density, sustainable, and cost-effective alternatives to lithium-ion batteries. Currently, several major challenges, such as a lack of suitable cathode materials and slow charge/discharge cycles, hinder their practical use. The process of positively charged ions incorporating into and moving through the cathode materials, a process known as cation diffusion, provides both functionality and routes to breakdown. Despite its critical nature, the fundamental steps and their impact on the host materials, such as spinel structured oxides, are not currently well understood. Researchers at Pacific Northwest National Laboratory (PNNL) used single crystalline Fe3O4 thin films grown on MgO as model system to study these fundamental cation diffusion processes.
The Impact
Cation diffusion plays a critical role in the functioning and failure of energy relevant technologies. However, determining what is happening in a material at an atomic scale is extremely challenging. By tightly controlling the synthesis and processing of magnesium and iron oxide films, the researchers found that Mg2+ diffusion and diffusion-induced phase transitions depend on whether or not O2 is present. Understanding cation diffusion in spinel structured oxides is particularly important as they are promising cathode materials for Mg ion batteries. The insights will help researchers develop new and better materials, as well as determine optimal operating conditions.
Summary
A team of researchers led by scientists from PNNL, investigated Mg2+ diffusion and diffusion induced phase transition processes in single crystalline Fe3O4 thin films grown on MgO(001). They used molecular beam epitaxy, a technique that gives scientists precise control over interface structure and thickness, to fabricate well-defined Fe3O4/MgO structures. To understand the evolution of the Fe3O4, the researchers thermally annealed the materials to promote cation diffusion and tracked structural transitions using high-resolution X-ray photoelectron spectroscopy and atomic-scale transmission electron microscopy. They found that annealing in oxygen leads to facile Mg2+ diffusion into Fe3O4 where Mg2+ can occupy the previous Fe2+ sites, leading to a phase similar to Fe3O4. In comparison, annealing in vacuum leads to the formation of an interfacial layer with a structure like MgO that blocks subsequent Mg2+ diffusion. This work highlights how the controlled synthesis of model material systems can provide fundamental insights into the directed growth of interfaces with tunable multivalent charge transfer processes, a major science goal of Joint Center for Energy Storage Research. While the thermal-induced diffusion mechanism differs from electrochemical diffusion mechanisms, the structure, defects, and properties of the resultant materials are expected to be similar, making these results relevant for future materials and structure design.
PNNL Contact
Yingge Du, Physical Sciences Division, Pacific Northwest National Laboratory, yingge.du@pnnl.gov
Funding
The work is supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Early Career Research Program under award no. 68278. The XPS characterization and a portion of the film growth activities were supported by the Joint Center for Energy Storage Research, an Energy Innovation Hub funded by the U.S. DOE Basic Energy Sciences program. A portion of the work was performed at the W. R. Wiley Environmental Molecular Sciences Laboratory, a DOE User Facility sponsored by the Office of Biological and Environmental Research program. We also thank the Singapore Synchrotron Light Source for providing the facilities support.
Published: November 25, 2020
L. W. Wangoh, Z. Yang, L. Wang, M. E. Bowden, X. Yin, A. T. S. Wee, K. T. Mueller, V. Murugesan, and Y. Du. 2020. "Mg2+ Diffusion-Induced Structural and Property Evolution in Epitaxial Fe3O4 Thin Films." ACS Nano, ASAP, [DOI: 10.1021/acsnano.0c04025]