Establishing Novel Carbon Dioxide Chemistry through Nano-Clustering in Water-Lean Solvents
Water-lean solvents form clusters that drive new reaction pathways unavailable to other solvents
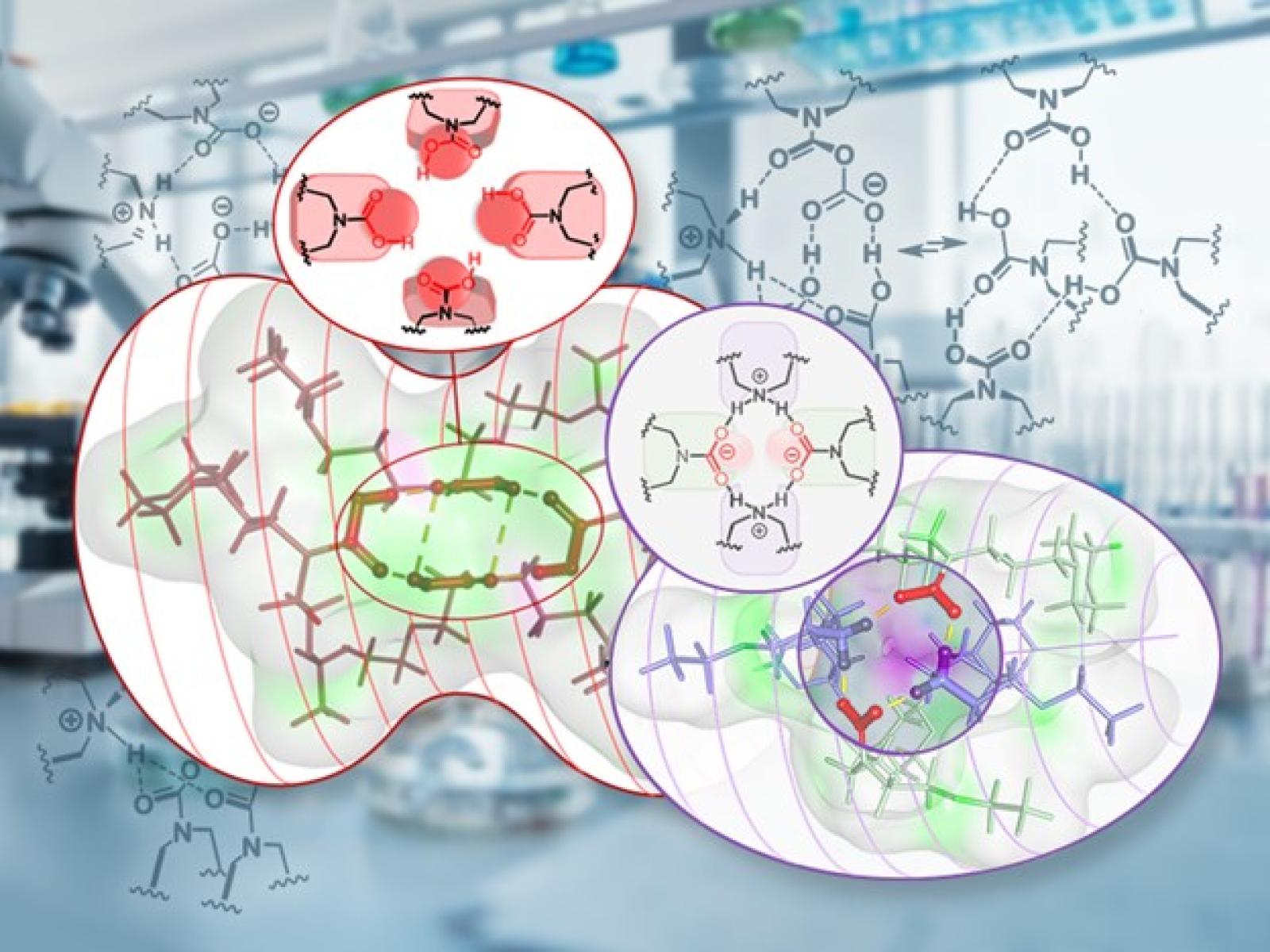
Water-lean solvents form nano-sized micelles that enable unique reactivity and computationally derived structures show the tetrameric clusters and their packing modes.
(Image by Michael Perkins | Pacific Northwest National Laboratory)
The Science
Capturing carbon dioxide (CO2) is a critical piece of reaching net-zero carbon emissions goals. Water-lean solvents have been previously shown to effectively capture CO2 and new work identifies the unique chemistry possible with these solvents. Researchers observed the formation of micellar-like nano-size clusters of four solvent molecules. The inside of these clusters has an active site core, similar to enzymes, that enables the formation of CO2-containing species by mechanisms not previously seen in carbon capture solvents. This represents a potential paradigm shift for the reactivity possible in carbon capture solvents.
The Impact
Reaching global net-zero carbon goals requires scientific innovation. This work opens a new focus in designing solvents for carbon capture. The discovery of the unique reactivities enabled by the solvent clusters can lead to new design principles that move beyond single carbon capture. The cooperativity and reactivity observed in this work may help researchers develop more effective and efficient carbon capture solvents, leading to a net-zero future.
Summary
Carbon capture, utilization, and storage is a key yet cost-intensive technology for the fight against climate change. In a combined experimental and modelling study of a single-component water-lean solvent, researchers found that CO2 capture is accompanied by the self-assembly of reverse micelle-like tetrameric clusters in solution. This spontaneous aggregation leads to stepwise capture phenomena with highly contrasting kinetic and thermodynamic features. The emergence of well-defined supramolecular architectures displaying an H-bonded internal core, reminiscent of enzymatic active sites, enables the formation of unprecedented CO2-containing molecular species such as carbamic acid, carbamic-anhydride, and alkoxy carbamic anhydrides. This dynamic system based on a single absorbent and CO2 extends the scope of adducts and mechanisms observed during carbon capture. It opens the way to new materials with a higher CO2 storage capacity and potential cooperative binding and also provides a means for carbamates to potentially act as initiators for future oligomerization or polymerization of CO2.
Contact
David Heldebrandt, Pacific Northwest National Laboratory, david.heldebrant@pnnl.gov
Funding
French authors were supported by the LABEX iMUST of the University of Lyon (ANR-10-LABX-0064), created within the Plan France 2030 set up by the French government and managed by the French National Research Agency. American authors acknowledge support from the Department of Energy (DOE), Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science user facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231.
Published: May 29, 2024
Leclaire, J., D. Heldebrant, et al. 2024. “Tetrameric self-assembling of water-lean solvents enables carbamate anhydride-based CO2 capture chemistry.” Nature Chemistry, DOI: 10.1038/s41557-024-01495-z