Discovering the Chemical Processes that Underpin Aluminum Solubility in Nuclear Waste
Fundamental chemistry paves the way for more efficient aluminum industrial processing
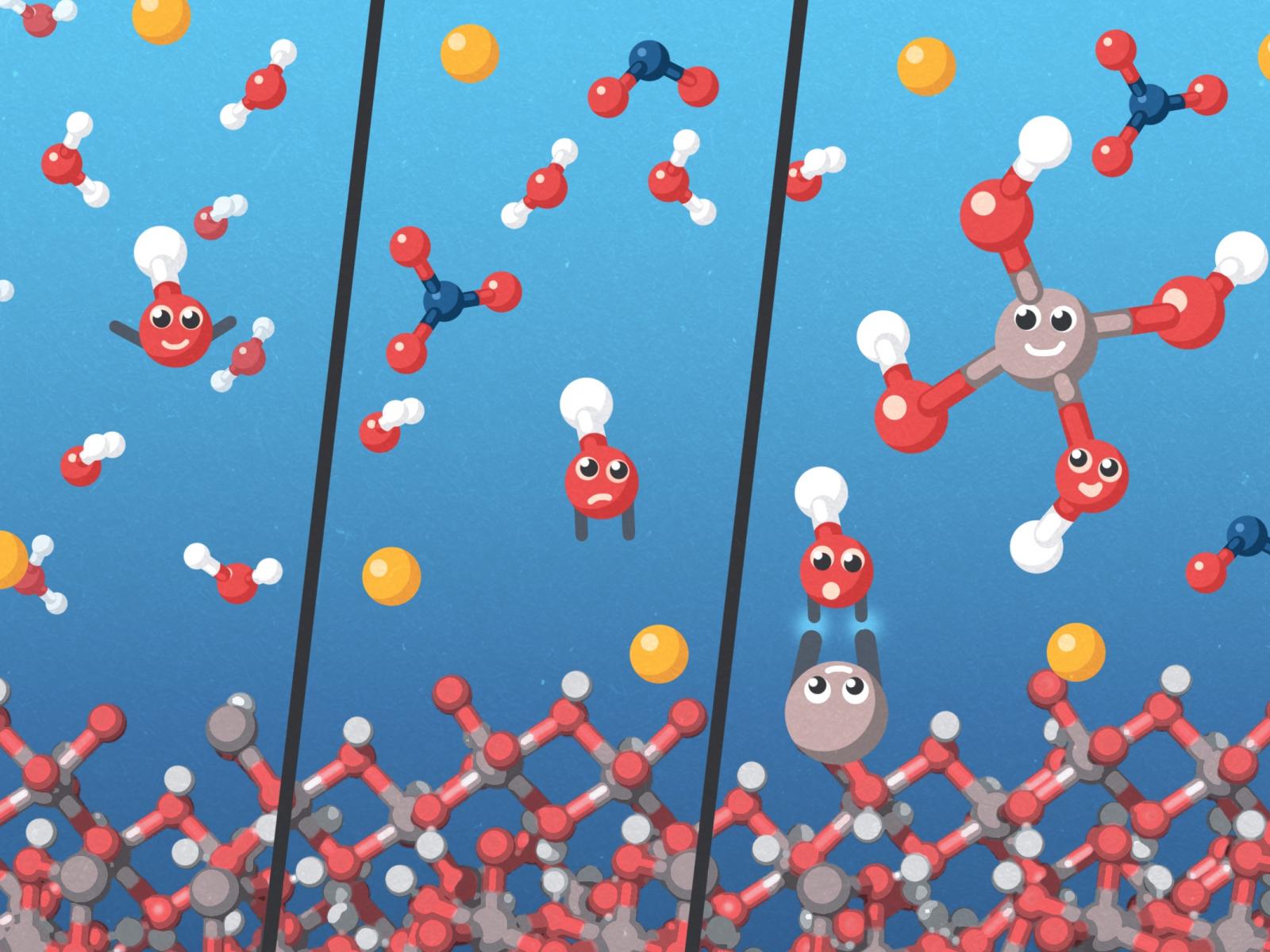
Dissolved nitrate and nitrite ions tie up solvent water, making it less available to solvate hydroxide. This situation causes the hydroxide to be more “chemically active” toward the aluminate ions from gibbsite, resulting in higher-than-expected concentrations of dissolved aluminate.
(Illustration by Rose Perry | Pacific Northwest National Laboratory)
The Science
Caustic, highly radioactive waste is currently stored in 177 underground tanks at the U.S. Department of Energy’s Hanford Site. Understanding the fundamental chemistry of the unique mixture in each tank can aid in efficiently retrieving and processing the waste.
The tank slurries contain high amounts of aluminum from Cold War-era nuclear processing systems present in the form of aluminum (oxy)hydroxides solids, gibbsite and boehmite, and as aluminate ions in solution.
It has long been known that sodium hydroxide dissolves gibbsite. But in Hanford tank waste, larger amounts of dissolved gibbsite are present than expected for a given concentration of sodium hydroxide. While models describing the thermodynamic behavior can be developed to predict aluminum solubility using the sodium hydroxide concentration, in highly concentrated caustic aqueous Hanford tank waste, the presence of other ions—such as sodium nitrate and nitrite—make the models more complex with more parameters. These parameters obscure the nature of the underlying molecular processes that cause elevated aluminum solubility.
Research conducted by scientists in the Interfacial Dynamics in Radioactive Environments and Materials (IDREAM) Energy Frontier Research Center and staff at Washington River Protection Solutions (WRPS) has revealed insights about why aluminum concentrations in Hanford tank waste are high and about the molecular processes involved in the dissolution of the gibbsite solids.
The Impact
As consumer and industrial demand for aluminum increases, more efficient aluminum production processes are needed because the current method is restricted by the slow precipitation of gibbsite. This study provided foundational chemistry to help potentially improve and expedite that process in the future. Beyond the aluminum industry, this study has applications to high-level radioactive waste processing. Understanding the fundamental chemistry of the unique mixture in each high-level radioactive waste storage tank allows for more efficient retrieval and waste processing.
Summary

Slurries in Hanford tanks contain high amounts of aluminum due to the chemical removal of nuclear fuel cladding, the addition of aluminum compounds as extracting agents, and the use of aluminum compounds to sequester fluoride. Aside from sodium hydroxide and aluminum, sodium nitrite and sodium nitrate are also highly prevalent in the tank waste.
This research provided a detailed understanding of the molecular processes underlying gibbsite solubility in sodium hydroxide, in solution with sodium nitrate and sodium nitrite. Researchers found that dissolved ions such as sodium, nitrate, and nitrite affect the extent of interaction between the hydroxide ion and gibbsite, resulting in increased aluminum solubility.
First, the dissolved sodium, nitrate, and nitrite ions tie up solvent water, making it less available to solvate hydroxide. This situation causes the hydroxide to be more “chemically active” toward the aluminate ions from gibbsite, resulting in higher-than-expected concentrations of dissolved aluminate. Second, the supplemental sodium, nitrate, and nitrite ions further stabilize aluminate by ion-ion interactions.
What’s Next
More research is needed to fully unravel the ion pairing interactions that dominate these complex low-water, concentrated electrolytes. In addition, further modeling work is needed to account for the behavior of these ions and their interactions in low-water environments.
Sponsors
IDREAM (Interfacial Dynamics in Radioactive Environments and Materials) is an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE) Office of Science, Basic Energy Sciences. Washington River Protection Solutions supported work that provided the samples for analysis. Raman spectroscopy was performed using the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research located at Pacific Northwest National Laboratory.
Research Team
Mateusz Dembowski, Michelle Snyder, Trent Graham, Ian Leavy, Steven Baum, Odeta Qafoku, Matthew Fountain, Kevin Rosso, Sue Clark, and Carolyn Pearce (PNNL); Calvin Delegard (Trade Wind Services); Jacob Reynolds (Washington River Protection Solutions); and Hsiu-Wen Wang (Oak Ridge National Laboratory).
The team’s finding, “Ion–ion interactions enhance aluminum solubility in alkaline suspensions of nano-gibbsite (α-Al(OH)3) with sodium nitrite/nitrate,” was published on the cover of the February 2020 edition of Physical Chemistry Chemical Physics (DOI: 10.1039/c9cp05856g).
Published: April 28, 2021
M. Dembowski, M.M. Snyder, C.H. Delegard, J.G. Reynolds, T.R. Graham, H. Wang, I.I. Leavy, S.R. Baum, O. Qafoku, M.S. Fountain, K.M. Russo, S.B. Clark, and C.I. Pearce. "Ion–ion interactions enhance aluminum solubility in alkaline suspensions of nano-gibbsite (α-Al(OH)3) with sodium nitrite/nitrate." Journal of Physical Chemistry Chemical Physics, 2020, 22, 4368-4378. [DOI: 10.1039/c9cp05856g].