Directing Hydrogen for Bond Breaking
Converting qualitative trends into quantitative knowledge
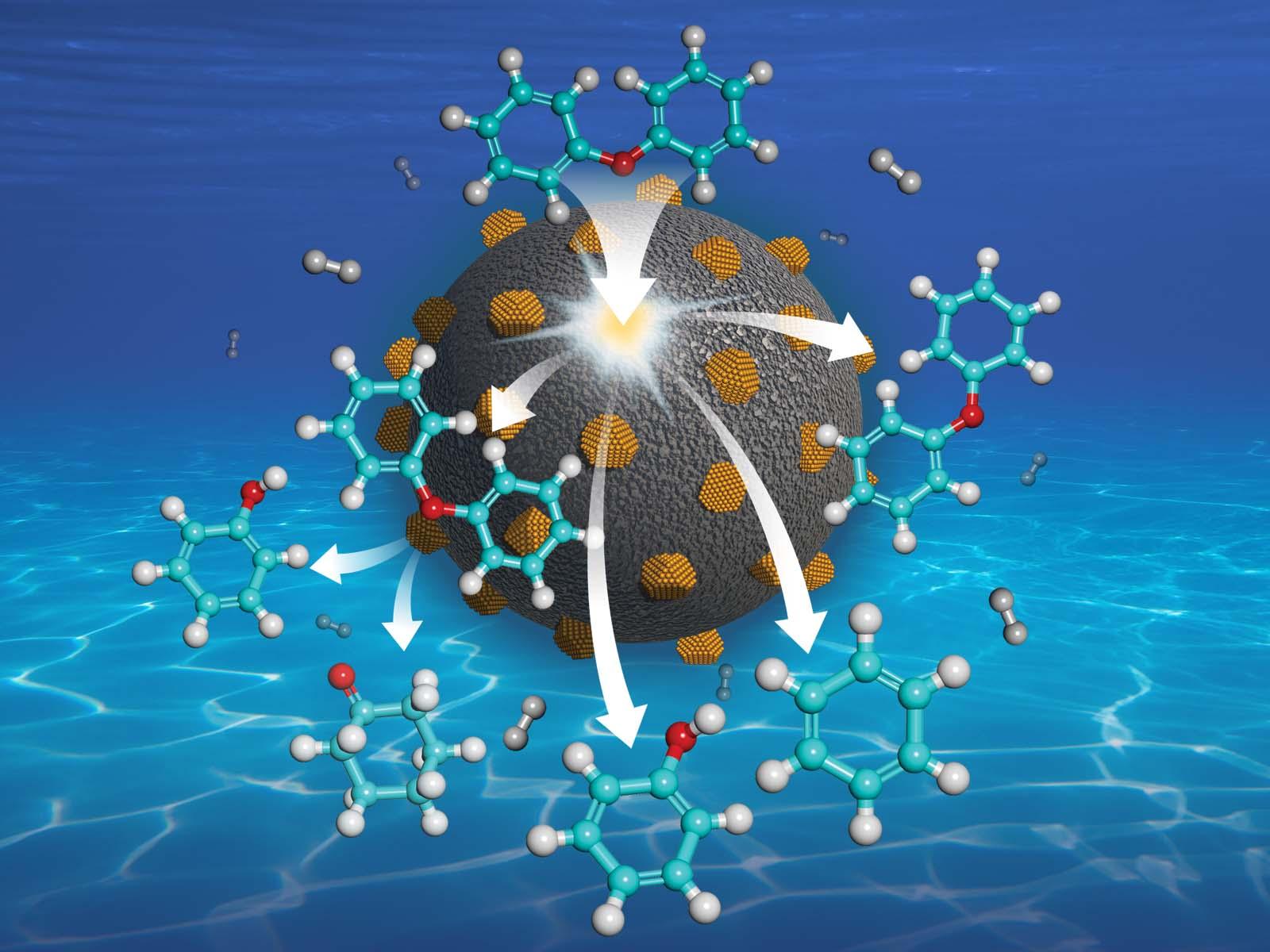
Platinum-group metals add hydrogen to molecules in multiple ways, which can be tuned by changing the reaction environment.
(Illustration by Mike Perkins | Pacific Northwest National Laboratory)
Breaking chemical bonds can be hard to do. In complicated molecules, as in biofuels or pharmaceuticals, reactions often need to target a single type of bond. Hydrogenation, or adding hydrogen to a molecule, is extremely important. However, it is difficult to do selectively. Hydrogen often adds across multiple types of bonds, either breaking apart a molecule into too many pieces or eliminating valuable chemical groups.
Researchers at Pacific Northwest National Laboratory (PNNL) first created a series of supported metal catalysts in a paper recently published in Angewandte Chemie. They then studied how each catalyst added hydrogen to diphenyl ether under a range of conditions. In diphenyl ether, hydrogen can either break a carbon-carbon bond or a carbon-oxygen bond. Selectively breaking carbon-oxygen bonds is an important step in effectively breaking down biomass.
“Generally, most papers either examine a single catalyst for multiple reactions or explore multiple catalysts for the same reaction,” said Oliver Gutiérrez-Tinoco, a PNNL chemist and author of the study. “Our approach lets us make direct, quantitative comparisons across different catalysis-relevant metals. We studied them under the same conditions and looked at reactions.”
The researchers studied catalysts made of ruthenium, rhodium, palladium, iridium, and platinum. They tested the catalysts in different conditions, changing the solvent and amount of hydrogen available. As expected, they found that the metals had different amounts of hydrogen on their surface. The amount of hydrogen made an important difference to the route the reactions preferentially took. However, the mechanisms were the same for all the metals across all the routes.
Using differences and the environment to change reactivity
The common reaction pathways all start with at least one hydrogen adding to a carbon-carbon bond in the diphenyl ether ring. Then, things diverge based on the environment and metal. Metals that strongly interact with hydrogen favored adding more hydrogen to carbon-carbon bonds. Metals with weak hydrogen interactions were more likely to break the carbon-oxygen bond.
While each metal has a natural affinity for hydrogen, the environment can also influence the reaction. Hydrogenolysis, breaking the C-O bond without water involved, takes more energy and depends less on how much hydrogen is around. Therefore, increasing the temperature and decreasing the amount of hydrogen pushes the system towards hydrogenolysis. Through these sorts of changes, researchers were able to find a system that broke the C-O bond with 95 percent selectivity.
Environmental tuning is a powerful tool in catalysis. Under the original conditions ruthenium, the metal at the heart of the 95 percent selective system, was 69 percent selective for another reaction. The broad comparisons in this work outline ways to push a metal-based system toward breaking a C-O bond.
“Understanding the way reaction conditions affect selectivity allows us to favor one reaction over another by tweaking the environment,” said Battelle fellow and PNNL Chemist Johannes Lercher. “We now have general strategies to push for breaking C-O bonds in these systems.”
This research was funded by the Department of Energy, Basic Energy Sciences program. In addition to Gutiérrez-Tinoco and Lercher, the PNNL team included Julian Schmid, Meng Wang, Morris Bullock, and Donald Camaioni.
Published: June 24, 2022